Companion animals: Translational scientist's new best friends
- PMID: 26446953
- PMCID: PMC4806851
- DOI: 10.1126/scitranslmed.aaa9116
Companion animals: Translational scientist's new best friends
Abstract
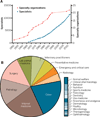
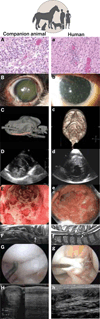
Knowledge and resources derived from veterinary medicine represent an underused resource that could serve as a bridge between data obtained from diseases models in laboratory animals and human clinical trials. Naturally occurring disease in companion animals that display the defining attributes of similar, if not identical, diseases in humans hold promise for providing predictive proof of concept in the evaluation of new therapeutics and devices. Here we outline comparative aspects of naturally occurring diseases in companion animals and discuss their current uses in translational medicine, benefits, and shortcomings. Last, we envision how these natural models of disease might ultimately decrease the failure rate in human clinical trials and accelerate the delivery of effective treatments to the human clinical market.
Copyright © 2015, American Association for the Advancement of Science.
Figures
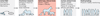
References
-
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40–51. - PubMed
-
- Code of Federal Regulations - Title 21 - Food and Drugs. 2015 312.23 (a)(8) www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=312.23.
-
- Guidance for industry: Preclinical assessment of investigational cellular and gene therapy products. Washington, DC: FDA, Center for Biologics Evaluation and Research; 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

