Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms
- PMID: 26447211
- PMCID: PMC4698403
- DOI: 10.1152/ajpregu.00178.2015
Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms
Abstract
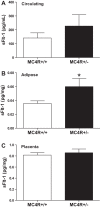
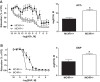
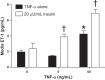
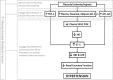
Preeclampsia (PE) is a pregnancy-specific disorder typically presenting as new-onset hypertension and proteinuria. While numerous epidemiological studies have demonstrated that obesity increases the risk of PE, the mechanisms have yet to be fully elucidated. Growing evidence from animal and human studies implicate placental ischemia in the etiology of this maternal syndrome. It is thought that placental ischemia is brought about by dysfunctional cytotrophoblast migration and invasion into the uterus and subsequent lack of spiral arteriole widening and placental perfusion. Placental ischemia/hypoxia stimulates the release of soluble placental factors into the maternal circulation where they cause endothelial dysfunction, particularly in the kidney, to elicit the clinical manifestations of PE. The most recognized of these factors are the anti-angiogenic sFlt-1 and pro-inflammatory TNF-α and AT1-AA, which promote endothelial dysfunction by reducing levels of the provasodilator nitric oxide and stimulating production of the potent vasoconstrictor endothelin-1 and reactive oxygen species. We hypothesize that obesity-related metabolic factors increase the risk for developing PE by impacting various stages in the pathogenesis of PE, namely, 1) cytotrophoblast migration and placental ischemia; 2) release of soluble placental factors into the maternal circulation; and 3) maternal endothelial and vascular dysfunction. This review will summarize the current experimental evidence supporting the concept that obesity and metabolic factors like lipids, insulin, glucose, and leptin affect placental function and increase the risk for developing hypertension in pregnancy by reducing placental perfusion; enhancing placental release of soluble factors; and by increasing the sensitivity of the maternal vasculature to placental ischemia-induced soluble factors.
Keywords: RUPP; body mass index; inflammation; placental ischemia; pregnancy; sFlt-1.
Copyright © 2015 the American Physiological Society.
Figures




References
-
- Ajne G, Nisell H, Jansson T. Effects of blockade of the endothelin receptor A and inhibition of nitric oxide synthesis on uteroplacental and renal blood flow in awake pregnant rats. Am J Obstet Gynecol 192: 295–301, 2005. - PubMed
-
- Akyol A, Langley-Evans SC, McMullen S. Obesity induced by cafeteria feeding and pregnancy outcome in the rat. Br J Nutr 102: 1601–1610, 2009. - PubMed
-
- Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002. - PubMed
-
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. - PubMed
-
- Athanassiades A, Hamilton GS, Lala PK. Vascular endothelial growth factor stimulates proliferation but not migration or invasiveness in human extravillous trophoblast. Biol Reprod 59: 643–654, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

