Constitutive Tor2 Activity Promotes Retention of the Amino Acid Transporter Agp3 at Trans-Golgi/Endosomes in Fission Yeast
- PMID: 26447710
- PMCID: PMC4598100
- DOI: 10.1371/journal.pone.0139045
Constitutive Tor2 Activity Promotes Retention of the Amino Acid Transporter Agp3 at Trans-Golgi/Endosomes in Fission Yeast
Abstract
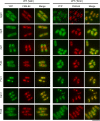
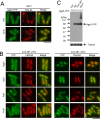
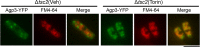
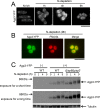
Amino acid transporters are located at specific subcellular compartments, and their localizations are regulated by the extracellular availability of amino acids. In yeast, target of rapamycin (TOR) activation induces the internalization of amino acid transporters located at the plasma membrane. However, whether and how TOR signaling regulates other amino acid transporters located at intracellular compartments remains unknown. Here, we demonstrate that in the fission yeast, the TOR inhibitor Torin-1 induces the transfer of several yellow fluorescent protein (YFP)-fused intracellular amino acid transporters, including Agp3, Isp5, Aat1, and Put4, from trans-Golgi/endosomes into the vacuoles. By contrast, the localizations of YFP-fused Can1, Fnx1, and Fnx2 transporter proteins were unaffected upon Torin-1 treatment. There are two TOR isoforms in fission yeast, Tor1 and Tor2. Whereas tor1 deletion did not affect the Torin-1-induced transfer of Agp3-YFP, Tor2 inhibition using a temperature-sensitive mutant induced the transfer of Agp3-YFP to the vacuolar lumen, similar to the effects of Torin-1 treatment. Tor2 inhibition also induced the transfer of the YFP-fused Isp5, Aat1, and Put4 transporter proteins to the vacuoles, although only partial transfer of the latter two transporters was observed. Under nitrogen depletion accompanied by reduced Tor2 activity, Agp3-YFP was transferred from the trans-Golgi/endosomes to the plasma membrane and then to the vacuoles, where it was degraded by the vacuolar proteases Isp6 and Psp3. Mutants with constitutively active Tor2 showed delayed transfer of Agp3-YFP to the plasma membrane upon nitrogen depletion. Cells lacking Tsc2, a negative regulator of Tor2, also showed a delay in this process in a Tor2-dependent manner. Taken together, these findings suggest that constitutive Tor2 activity is critical for the retention of amino acid transporters at trans-Golgi/endosomes. Moreover, nitrogen depletion suppresses Tor2 activity through Tsc2, thereby promoting the surface expression of these transporters.
Conflict of interest statement
Figures


Similar articles
-
Tor Signaling Regulates Transcription of Amino Acid Permeases through a GATA Transcription Factor Gaf1 in Fission Yeast.PLoS One. 2015 Dec 21;10(12):e0144677. doi: 10.1371/journal.pone.0144677. eCollection 2015. PLoS One. 2015. PMID: 26689777 Free PMC article.
-
The fission yeast β-arrestin-like protein Any1 is involved in TSC-Rheb signaling and the regulation of amino acid transporters.J Cell Sci. 2013 Sep 1;126(Pt 17):3972-81. doi: 10.1242/jcs.128355. Epub 2013 Jun 26. J Cell Sci. 2013. PMID: 23813957
-
Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast.Genetics. 2007 Mar;175(3):1153-62. doi: 10.1534/genetics.106.064170. Epub 2006 Dec 18. Genetics. 2007. PMID: 17179073 Free PMC article.
-
TOR signaling in fission yeast.Crit Rev Biochem Mol Biol. 2008 Jul-Aug;43(4):277-83. doi: 10.1080/10409230802254911. Crit Rev Biochem Mol Biol. 2008. PMID: 18756382 Review.
-
The TSC/Rheb/TOR signaling pathway in fission yeast and mammalian cells: temperature sensitive and constitutive active mutants of TOR.Cell Cycle. 2007 Jul 15;6(14):1692-5. doi: 10.4161/cc.6.14.4478. Epub 2007 May 21. Cell Cycle. 2007. PMID: 17637564 Review.
Cited by
-
Analysis of the SNARE Stx8 recycling reveals that the retromer-sorting motif has undergone evolutionary divergence.PLoS Genet. 2021 Mar 31;17(3):e1009463. doi: 10.1371/journal.pgen.1009463. eCollection 2021 Mar. PLoS Genet. 2021. PMID: 33788833 Free PMC article.
-
The Loss of Lam2 and Npr2-Npr3 Diminishes the Vacuolar Localization of Gtr1-Gtr2 and Disinhibits TORC1 Activity in Fission Yeast.PLoS One. 2016 May 26;11(5):e0156239. doi: 10.1371/journal.pone.0156239. eCollection 2016. PLoS One. 2016. PMID: 27227887 Free PMC article.
-
Interplays of AMPK and TOR in Autophagy Regulation in Yeast.Cells. 2023 Feb 4;12(4):519. doi: 10.3390/cells12040519. Cells. 2023. PMID: 36831186 Free PMC article. Review.
-
Environmental control of Pub1 (NEDD4 family E3 ligase) in Schizosaccharomyces pombe is regulated by TORC2 and Gsk3.Life Sci Alliance. 2022 Feb 4;5(5):e202101082. doi: 10.26508/lsa.202101082. Print 2022 May. Life Sci Alliance. 2022. PMID: 35121625 Free PMC article.
-
The leucine-NH4+ uptake regulator Any1 limits growth as part of a general amino acid control response to loss of La protein by fission yeast.PLoS One. 2021 Jun 21;16(6):e0253494. doi: 10.1371/journal.pone.0253494. eCollection 2021. PLoS One. 2021. PMID: 34153074 Free PMC article.
References
-
- Nakase Y, Nakase M, Kashiwazaki J, Murai T, Otsubo Y, Mabuchi I, et al. The fission yeast beta-arrestin-like protein Any1 is involved in TSC-Rheb signaling and the regulation of amino acid transporters. Journal of cell science. 2013;126(Pt 17):3972–81. doi: 10.1242/jcs.128355. 23813957 - DOI - PubMed
-
- Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of cell science. 2009;122(Pt 20):3589–94. doi: 10.1242/jcs.051011. 19812304 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

