A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3-year results
- PMID: 26448178
- PMCID: PMC4647871
- DOI: 10.1038/bjc.2015.313
A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3-year results
Abstract
Background: This exploratory study evaluated the safety/efficacy of nintedanib or sunitinib as first-line therapy in patients with advanced renal cell carcinoma (RCC).
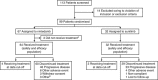
Methods: Ninety-six patients were randomised (2:1) to either nintedanib (200 mg twice daily) or sunitinib (50 mg kg(-1) once daily (4 weeks on treatment; 2 weeks off)). Primary endpoint was progression-free survival (PFS) at 9 months. P-values reported are descriptive only; the study was not powered for such comparisons.
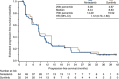
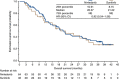
Results: Progression-free survival at 9 months was comparable between nintedanib and sunitinib (43.1% vs 45.2%, respectively; P=0.85). Median PFS was 8.4 months in each group (hazard ratio (HR), 1.12; 95% confidence interval (CI): 0.70-1.80; P=0.64). Median overall survival was 20.4 and 21.2 months for nintedanib and sunitinib, respectively (HR, 0.92; 95% CI: 0.54-1.56; P=0.76). Overall incidence of any grade adverse events (AEs) was comparable (90.6% vs 93.8%); AEs grade ⩾ 3 were lower with nintedanib than sunitinib (48.4% vs 59.4%). Nintedanib was associated with lower incidences of some AEs typical of antiangiogenic tyrosine kinase inhibitors (TKIs): hypertension, hypothyroidism, hand-foot syndrome, cardiac disorders and haematological abnormalities.
Conclusions: In patients with advanced RCC, nintedanib has promising efficacy and similar tolerability to sunitinib, and a manageable safety profile with fewer TKI-associated AEs.
Figures
References
-
- Boehringer Ingelheim (2014. a) Ofev® (nintedanib) prescribing information [Online]. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2014/205832s000lbl.pdf (accessed on April 2015).
-
- Boehringer Ingelheim (2014. b) Vargatef® (nintedanib) summary of product characteristics [Online]. Available at: www.ema.europa.eu (accessed on April 2015).
-
- Cancer Therapy Evaluation Program (2006) Common Terminology Criteria for Adverse Events Version 3.0 [Online] Available http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/... accessed September 2015.
-
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8: 299–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

