Identification of High-Risk Plaques by MRI and Fluorescence Imaging in a Rabbit Model of Atherothrombosis
- PMID: 26448434
- PMCID: PMC4598148
- DOI: 10.1371/journal.pone.0139833
Identification of High-Risk Plaques by MRI and Fluorescence Imaging in a Rabbit Model of Atherothrombosis
Abstract
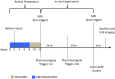
Introduction: The detection of atherosclerotic plaques at risk for disruption will be greatly enhanced by molecular probes that target vessel wall biomarkers. Here, we test if fluorescently-labeled Activatable Cell Penetrating Peptides (ACPPs) could differentiate stable plaques from vulnerable plaques that disrupt, forming a luminal thrombus. Additionally, we test the efficacy of a combined ACPP and MRI technique for identifying plaques at high risk of rupture.
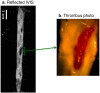
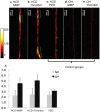
Methods and results: In an atherothrombotic rabbit model, disrupted plaques were identified with in vivo MRI and co-registered in the same rabbit aorta with the in vivo uptake of ACPPs, cleaved by matrix metalloproteinases (MMPs) or thrombin. ACPP uptake, mapped ex vivo in whole aortas, was higher in disrupted compared to non-disrupted plaques. Specifically, disrupted plaques demonstrated a 4.5~5.0 fold increase in fluorescence enhancement, while non-disrupted plaques showed only a 2.2~2.5 fold signal increase. Receiver operating characteristic (ROC) analysis indicates that both ACPPs (MMP and thrombin) show high specificity (84.2% and 83.2%) and sensitivity (80.0% and 85.7%) in detecting disrupted plaques. The detection power of ACPPs was improved when combined with the MRI derived measure, outward remodeling ratio.
Conclusions: Our targeted fluorescence ACPP probes distinguished disrupted plaques from stable plaques with high sensitivity and specificity. The combination of anatomic, MRI-derived predictors for disruption and ACPP uptake can further improve the power for identification of high-risk plaques and suggests future development of ACPPs with molecular MRI as a readout.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

