Adjunctive Aripiprazole Treatment for Risperidone-Induced Hyperprolactinemia: An 8-Week Randomized, Open-Label, Comparative Clinical Trial
- PMID: 26448615
- PMCID: PMC4598102
- DOI: 10.1371/journal.pone.0139717
Adjunctive Aripiprazole Treatment for Risperidone-Induced Hyperprolactinemia: An 8-Week Randomized, Open-Label, Comparative Clinical Trial
Abstract
Objective: The present study aimed to evaluate the efficacy and safety of adjunctive aripiprazole treatment in schizophrenia patients with risperidone-induced hyperprolactinemia.
Methods: One hundred and thirteen patients who were receiving a stable dose of risperidone were randomly assigned to either adjunctive aripiprazole treatment (10 mg/day) (aripiprazole group) or no additional treatment (control group) at a 1:1 ratio for 8 weeks. Schizophrenia symptoms were measured using the Positive and Negative Syndrome Scale (PANSS). Rating scales and safety assessments (RSESE, BARS, UKU) were performed at baseline and at weeks 4 and 8. Serum levels of prolactin were determined at baseline and at weeks 2, 4, 6 and 8. Metabolic parameters were determined at baseline and again at weeks 4 and 8.
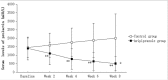
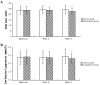
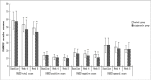
Results: One hundred and thirteen patients were enrolled in this study, and 107 patients completed the study (54 in the aripiprazole group, and 53 in the control group). PANSS-total scores in the aripiprazole group decreased significantly at week 4 (P = 0.003) and week 8 (P = 0.007) compared with the control group. PANSS-negative scores in the aripiprazole group also decreased significantly at week 4 (P = 0.005) and week 8 (P< 0.001) compared with the control group. Serum levels of prolactin in the aripiprazole group decreased significantly at week 2 (P< 0.001), week 4 (P< 0.001), week 6 (P< 0.001) and week 8 (P< 0.001) compared with the control group. There were no significant differences in changes of Fasting Plasma Glucose, Total cholesterol, Triglycerides and High Density Lipoprotein within each group at week 4 and 8 execpt low density lipoproteins. There was no significant difference in the incidence of adverse reactions between the two groups.
Conclusions: Adjunctive aripiprazole treatment may be beneficial in reducing serum levels of prolactin and improving negative symptoms in schizophrenia patients with risperidone-induced hyperprolactinemia.
Trial registration: chictr.org ChiCTR-IOR-15006278.
Conflict of interest statement
Figures
References
-
- Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology 2003;28 Suppl 2:55–68. - PubMed
-
- Leonard MP, Nickel CJ, Morales A. Hyperprolactinemia and impotence: why, when and how to investigate. The Journal of urology 1989;142:992–994. - PubMed
-
- Paulzen M, Grunder G. Amisulpride-induced hyperprolactinaemia is not reversed by addition of aripiprazole. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2007;10:149–151. - PubMed
-
- Leucht S, Barnes TR, Kissling W, Engel RR, Correll C, Kane JM. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. The American journal of psychiatry 2003;160:1209–1222. - PubMed
-
- Shim J-C, Shin J-G, Kelly D, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. American Journal of Psychiatry 2007;164:1404–1410. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

