Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis
- PMID: 26448760
- PMCID: PMC4581578
- DOI: 10.1155/2015/834893
Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis
Abstract
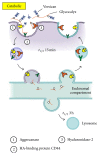
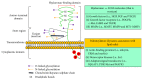
A wound is a type of injury that damages living tissues. In this review, we will be referring mainly to healing responses in the organs including skin and the lungs. Fibrosis is a process of dysregulated extracellular matrix (ECM) production that leads to a dense and functionally abnormal connective tissue compartment (dermis). In tissues such as the skin, the repair of the dermis after wounding requires not only the fibroblasts that produce the ECM molecules, but also the overlying epithelial layer (keratinocytes), the endothelial cells, and smooth muscle cells of the blood vessel and white blood cells such as neutrophils and macrophages, which together orchestrate the cytokine-mediated signaling and paracrine interactions that are required to regulate the proper extent and timing of the repair process. This review will focus on the importance of extracellular molecules in the microenvironment, primarily the proteoglycans and glycosaminoglycan hyaluronan, and their roles in wound healing. First, we will briefly summarize the physiological, cellular, and biochemical elements of wound healing, including the importance of cytokine cross-talk between cell types. Second, we will discuss the role of proteoglycans and hyaluronan in regulating these processes. Finally, approaches that utilize these concepts as potential therapies for fibrosis are discussed.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

