MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity
- PMID: 26448907
- PMCID: PMC4571540
- DOI: 10.1016/j.fob.2015.08.010
MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity
Abstract
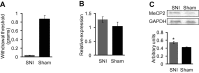
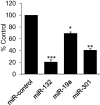
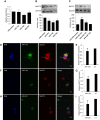
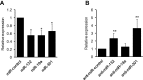
Nerve injury induces chronic pain and dysregulation of microRNAs in dorsal root ganglia (DRG). Several downregulated microRNAs are predicted to target Mecp2. MECP2 mutations cause Rett syndrome and these patients report decreased pain perception. We confirmed MeCP2 upregulation in DRG following nerve injury and repression of MeCP2 by miRNAs in vitro. MeCP2 regulates brain-derived neurotrophic factor (BDNF) and downregulation of MeCP2 by microRNAs decreased Bdnf in vitro. MeCP2 T158A mice exhibited reduced mechanical sensitivity and Mecp2-null and MeCP2 T158A mice have decreased Bdnf in DRG. MeCP2-mediated regulation of Bdnf in the DRG could contribute to altered pain sensitivity.
Keywords: +/Y, male wild-type littermate control for either MeCP2 T158A knock in mouse or Mecp2-null mouse; 3′UTR, three prime untranslated region; ATF3, activating transcription factor 3; BDNF; BDNF, brain derived neurotrophic factor; CFA, complete Freund’s adjuvant; DRG, dorsal root ganglia; L4/L5, 4th or 5th lumbar vertebra; MeCP2; MeCP2 T158A/Y, male MeCP2 T158A knock in mouse; MeCP2, methyl-CpG-binding protein 2; Neuropathic pain; RTT, Rett syndrome; SNI, spared nerve injury; T158A, threonine 158 conversion to alanine; TrkB, tropomyosin receptor kinase B; miRNA; −/Y, male Mecp2-null mouse.
Figures
References
-
- Mogil J.S., Davis K.D., Derbyshire S.W. The necessity of animal models in pain research. Pain. 2010;151:12–17. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

