Effect of Long-Term Treatment with Fimasartan on Transient Focal Ischemia in Rat Brain
- PMID: 26448932
- PMCID: PMC4584036
- DOI: 10.1155/2015/295925
Effect of Long-Term Treatment with Fimasartan on Transient Focal Ischemia in Rat Brain
Abstract
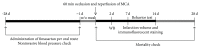
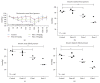
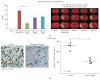
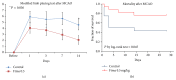
Fimasartan is a newly developed angiotensin receptor blocker, which may have protective effects during myocardial infarction or atherosclerosis. In this context, we investigated the effects of long-term treatment with low-dose fimasartan on focal ischemia in rat brain. We induced focal ischemia in brain by transient intraluminal occlusion of middle cerebral artery (MCA) and administered low-dose (0.5 mg/kg) or regular doses (1 or 3 mg/kg) of fimasartan via intravenous routes. After the administration of low-dose (0.5 mg/kg) fimasartan, blood pressure did not decrease compared to the phosphate-buffered saline- (PBS-) control with MCA occlusion (MCAO) group. The infarct volume and ischemic cell death were reduced in the low-dose fimasartan-treated group (46 ± 41 mm(3) for 0.5 mg/kg and 153 ± 47 mm(3) for PBS-control with MCAO; P < 0.01) but not in the regular-dose groups. Low-dose fimasartan treatment improved functional recovery after ischemia and significantly decreased mortality. In our study, fimasartan reduced the degradation of IκB and the formation of an inflammatory end-product, COX-2. As a result, the recruitment of inflammatory cells in the peri-infarct area decreased in fimasartan-treated group. We have demonstrated that long-term, low-dose fimasartan treatment improved outcomes after focal ischemia in the brain via a reduction of inflammation.
Figures
Similar articles
-
Pretreatment with low-dose fimasartan ameliorates NLRP3 inflammasome-mediated neuroinflammation and brain injury after intracerebral hemorrhage.Exp Neurol. 2018 Dec;310:22-32. doi: 10.1016/j.expneurol.2018.08.013. Epub 2018 Aug 29. Exp Neurol. 2018. PMID: 30171865 Free PMC article.
-
Pharmacological Profiles of a Highly Potent and Long-Acting Angiotensin II Receptor Antagonist, Fimasartan, in Rats and Dogs after Oral Administration.Biol Pharm Bull. 2017;40(7):992-1001. doi: 10.1248/bpb.b16-00987. Biol Pharm Bull. 2017. PMID: 28674263
-
Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury.Int J Cardiol. 2013 Oct 3;168(3):2851-9. doi: 10.1016/j.ijcard.2013.03.151. Epub 2013 May 1. Int J Cardiol. 2013. PMID: 23642815
-
Fimasartan: A New Angiotensin Receptor Blocker.Drugs. 2016 Jul;76(10):1015-22. doi: 10.1007/s40265-016-0592-1. Drugs. 2016. PMID: 27272555 Review.
-
PK/PD evaluation of fimasartan for the treatment of hypertension Current evidences and future perspectives.Expert Opin Drug Metab Toxicol. 2018 May;14(5):533-541. doi: 10.1080/17425255.2018.1468435. Epub 2018 May 10. Expert Opin Drug Metab Toxicol. 2018. PMID: 29676941 Review.
Cited by
-
Neuroprotective effect of angiotensin II receptor blockers on the risk of incident Alzheimer's disease: A nationwide population-based cohort study.Front Aging Neurosci. 2023 Mar 6;15:1137197. doi: 10.3389/fnagi.2023.1137197. eCollection 2023. Front Aging Neurosci. 2023. PMID: 36949774 Free PMC article.
-
Therapeutic potential of the renin angiotensin system in ischaemic stroke.Exp Transl Stroke Med. 2016 Oct 7;8:8. doi: 10.1186/s13231-016-0022-1. eCollection 2016. Exp Transl Stroke Med. 2016. PMID: 27761230 Free PMC article. Review.
-
Fimasartan, an angiotensin II receptor antagonist, ameliorates an in vivo zebrafish model of heart failure.Korean J Intern Med. 2020 Nov;35(6):1400-1410. doi: 10.3904/kjim.2019.038. Epub 2020 Mar 13. Korean J Intern Med. 2020. PMID: 32164398 Free PMC article.
-
Anti-inflammatory effects of fimasartan via Akt, ERK, and NFκB pathways on astrocytes stimulated by hemolysate.Inflamm Res. 2016 Feb;65(2):115-23. doi: 10.1007/s00011-015-0895-9. Epub 2015 Nov 25. Inflamm Res. 2016. PMID: 26608500
-
Central and cerebral haemodynamic changes after antihypertensive therapy in ischaemic stroke patients: A double-blind randomised trial.Sci Rep. 2018 Jan 24;8(1):1556. doi: 10.1038/s41598-018-19998-4. Sci Rep. 2018. PMID: 29367614 Free PMC article. Clinical Trial.
References
-
- Kim T. W., Yoo B. W., Lee J. K., et al. Synthesis and antihypertensive activity of pyrimidin-4(3H)-one derivatives as losartan analogue for new angiotensin II receptor type 1 (AT1) antagonists. Bioorganic and Medicinal Chemistry Letters. 2012;22(4):1649–1654. doi: 10.1016/j.bmcl.2011.12.116. - DOI - PubMed
-
- Lee J.-Y., Lee C. W., Kim W.-J., et al. Antiatherosclerotic effects of the novel angiotensin receptor antagonist fimasartan on plaque progression and stability in a rabbit model: a double-blind placebo-controlled trial. Journal of Cardiovascular Pharmacology. 2013;62(2):229–236. doi: 10.1097/fjc.0b013e318297458b. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

