Efficient Mitochondrial Genome Editing by CRISPR/Cas9
- PMID: 26448933
- PMCID: PMC4581504
- DOI: 10.1155/2015/305716
Efficient Mitochondrial Genome Editing by CRISPR/Cas9
Abstract
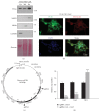
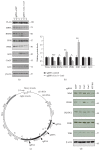
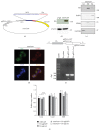
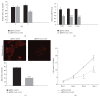
The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 system has been widely used for nuclear DNA editing to generate mutations or correct specific disease alleles. Despite its flexible application, it has not been determined if CRISPR/Cas9, originally identified as a bacterial defense system against virus, can be targeted to mitochondria for mtDNA editing. Here, we show that regular FLAG-Cas9 can localize to mitochondria to edit mitochondrial DNA with sgRNAs targeting specific loci of the mitochondrial genome. Expression of FLAG-Cas9 together with gRNA targeting Cox1 and Cox3 leads to cleavage of the specific mtDNA loci. In addition, we observed disruption of mitochondrial protein homeostasis following mtDNA truncation or cleavage by CRISPR/Cas9. To overcome nonspecific distribution of FLAG-Cas9, we also created a mitochondria-targeted Cas9 (mitoCas9). This new version of Cas9 localizes only to mitochondria; together with expression of gRNA targeting mtDNA, there is specific cleavage of mtDNA. MitoCas9-induced reduction of mtDNA and its transcription leads to mitochondrial membrane potential disruption and cell growth inhibition. This mitoCas9 could be applied to edit mtDNA together with gRNA expression vectors without affecting genomic DNA. In this brief study, we demonstrate that mtDNA editing is possible using CRISPR/Cas9. Moreover, our development of mitoCas9 with specific localization to the mitochondria should facilitate its application for mitochondrial genome editing.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

