Comprehensive Review of Adipose Stem Cells and Their Implication in Distraction Osteogenesis and Bone Regeneration
- PMID: 26448947
- PMCID: PMC4584039
- DOI: 10.1155/2015/842975
Comprehensive Review of Adipose Stem Cells and Their Implication in Distraction Osteogenesis and Bone Regeneration
Abstract
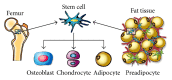
Bone is one of the most dynamic tissues in the human body that can heal following injury without leaving a scar. However, in instances of extensive bone loss, this intrinsic capacity of bone to heal may not be sufficient and external intervention becomes necessary. Several techniques are available to address this problem, including autogenous bone grafts and allografts. However, all these techniques have their own limitations. An alternative method is the technique of distraction osteogenesis, where gradual and controlled distraction of two bony segments after osteotomy leads to induction of new bone formation. Although distraction osteogenesis usually gives satisfactory results, its major limitation is the prolonged duration of time required before the external fixator is removed, which may lead to numerous complications. Numerous methods to accelerate bone formation in the context of distraction osteogenesis have been reported. A viable alternative to autogenous bone grafts for a source of osteogenic cells is mesenchymal stem cells from bone marrow. However, there are certain problems with bone marrow aspirate. Hence, scientists have investigated other sources for mesenchymal stem cells, specifically adipose tissue, which has been shown to be an excellent source of mesenchymal stem cells. In this paper, the potential use of adipose stem cells to stimulate bone formation is discussed.
Figures
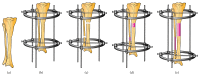




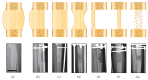
References
-
- Tsuchiya H. Treatment of segmental bone loss due to tumors. In: McCarthy R. H. A. J. J., editor. Management of Limb-Length Discrepancies. Rosemont, Ill, USA: American Academy of Orthopaedic Surgeons; 2011. pp. 173–178.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

