CLARITY and PACT-based imaging of adult zebrafish and mouse for whole-animal analysis of infections
- PMID: 26449262
- PMCID: PMC4728314
- DOI: 10.1242/dmm.021394
CLARITY and PACT-based imaging of adult zebrafish and mouse for whole-animal analysis of infections
Abstract
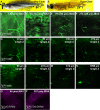
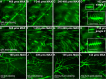
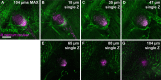
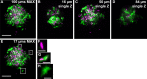
Visualization of infection and the associated host response has been challenging in adult vertebrates. Owing to their transparency, zebrafish larvae have been used to directly observe infection in vivo; however, such larvae have not yet developed a functional adaptive immune system. Cells involved in adaptive immunity mature later and have therefore been difficult to access optically in intact animals. Thus, the study of many aspects of vertebrate infection requires dissection of adult organs or ex vivo isolation of immune cells. Recently, CLARITY and PACT (passive clarity technique) methodologies have enabled clearing and direct visualization of dissected organs. Here, we show that these techniques can be applied to image host-pathogen interactions directly in whole animals. CLARITY and PACT-based clearing of whole adult zebrafish and Mycobacterium tuberculosis-infected mouse lungs enables imaging of mycobacterial granulomas deep within tissue to a depth of more than 1 mm. Using established transgenic lines, we were able to image normal and pathogenic structures and their surrounding host context at high resolution. We identified the three-dimensional organization of granuloma-associated angiogenesis, an important feature of mycobacterial infection, and characterized the induction of the cytokine tumor necrosis factor (TNF) within the granuloma using an established fluorescent reporter line. We observed heterogeneity in TNF induction within granuloma macrophages, consistent with an evolving view of the tuberculous granuloma as a non-uniform, heterogeneous structure. Broad application of this technique will enable new understanding of host-pathogen interactions in situ.
Keywords: CLARITY; Imaging; Infection; Mouse; Mycobacteria; PACT; Tuberculosis; Zebrafish.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

