Cystinosin is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling
- PMID: 26449607
- PMCID: PMC4884097
- DOI: 10.1681/ASN.2014090937
Cystinosin is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling
Abstract
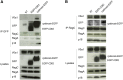
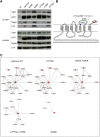
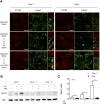
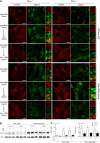
Cystinosis is a rare autosomal recessive storage disorder characterized by defective lysosomal efflux of cystine due to mutations in the CTNS gene encoding the lysosomal cystine transporter, cystinosin. Lysosomal cystine accumulation leads to crystal formation and functional impairment of multiple organs. Moreover, cystinosis is the most common inherited cause of renal Fanconi syndrome in children. Oral cysteamine therapy delays disease progression by reducing intracellular cystine levels. However, because cysteamine does not correct all complications of cystinosis, including Fanconi syndrome, we hypothesized that cystinosin could have novel roles in addition to transporting cystine out of the lysosome. By coimmunoprecipitation experiments and mass spectrometry, we found cystinosin interacts with almost all components of vacuolar H(+)-ATPase and the Ragulator complex and with the small GTPases Ras-related GTP-binding protein A (RagA) and RagC. Furthermore, the mammalian target of rapamycin complex 1 (mTORC1) pathway was downregulated in proximal tubular cell lines derived from Ctns(-/-) mice. Decrease of lysosomal cystine levels by cysteamine did not rescue mTORC1 activation in these cells, suggesting that the downregulation of mTORC1 is due to the absence of cystinosin rather than to the accumulation of cystine. Our results show a dual role for cystinosin as a cystine transporter and as a component of the mTORC1 pathway, and provide an explanation for the appearance of Fanconi syndrome in cystinosis. Furthermore, this study highlights the need to develop new treatments not dependent on lysosomal cystine depletion alone for this devastating disease.
Keywords: Fanconi syndrome; cystinosin; cystinosis; mTORC1.
Copyright © 2016 by the American Society of Nephrology.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

