Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: individual patient data meta-analysis
- PMID: 26449630
- PMCID: PMC4599727
- DOI: 10.1186/s12885-015-1664-4
Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: individual patient data meta-analysis
Abstract
Background: Magnetic resonance imaging (MRI) may guide breast cancer surgery by measuring residual tumor size post-neoadjuvant chemotherapy (NAC). Accurate measurement may avoid overly radical surgery or reduce the need for repeat surgery. This individual patient data (IPD) meta-analysis examines MRI's agreement with pathology in measuring the longest tumor diameter and compares MRI with alternative tests.
Methods: A systematic review of MEDLINE, EMBASE, PREMEDLINE, Database of Abstracts of Reviews of Effects, Heath Technology Assessment, and Cochrane databases identified eligible studies. Primary study authors supplied IPD in a template format constructed a priori. Mean differences (MDs) between tests and pathology (i.e. systematic bias) were calculated and pooled by the inverse variance method; limits of agreement (LOA) were estimated. Test measurements of 0.0 cm in the presence of pathologic residual tumor, and measurements >0.0 cm despite pathologic complete response (pCR) were described for MRI and alternative tests.
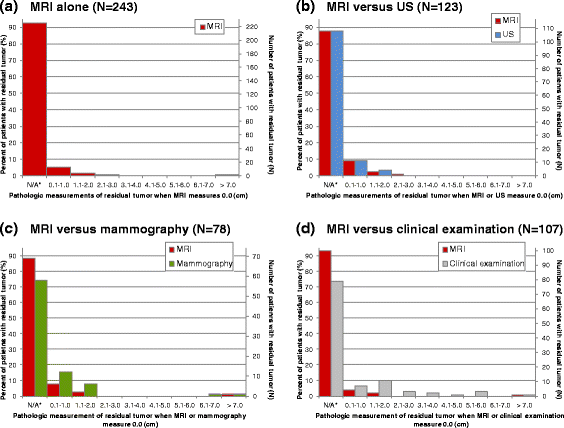
Results: Eight studies contributed IPD (N = 300). The pooled MD for MRI was 0.0 cm (LOA: +/-3.8 cm). Ultrasound underestimated pathologic size (MD: -0.3 cm) relative to MRI (MD: 0.1 cm), with comparable LOA. MDs were similar for MRI (0.1 cm) and mammography (0.0 cm), with wider LOA for mammography. Clinical examination underestimated size (MD: -0.8 cm) relative to MRI (MD: 0.0 cm), with wider LOA. Tumors "missed" by MRI typically measured 2.0 cm or less at pathology; tumors >2.0 cm were more commonly "missed" by clinical examination (9.3 %). MRI measurements >5.0 cm occurred in 5.3 % of patients with pCR, but were more frequent for mammography (46.2 %).
Conclusions: There was no systematic bias in MRI tumor measurement, but LOA are large enough to be clinically important. MRI's performance was generally superior to ultrasound, mammography, and clinical examination, and it may be considered the most appropriate test in this setting. Test combinations should be explored in future studies.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

