Three-Dimensional Vascular Network Assembly From Diabetic Patient-Derived Induced Pluripotent Stem Cells
- PMID: 26449749
- PMCID: PMC4603427
- DOI: 10.1161/ATVBAHA.115.306362
Three-Dimensional Vascular Network Assembly From Diabetic Patient-Derived Induced Pluripotent Stem Cells
Abstract
Objective: In diabetics, hyperglycemia results in deficient endothelial progenitors and cells, leading to cardiovascular complications. We aim to engineer 3-dimensional (3D) vascular networks in synthetic hydrogels from type 1 diabetes mellitus (T1D) patient-derived human-induced pluripotent stem cells (hiPSCs), to serve as a transformative autologous vascular therapy for diabetic patients.
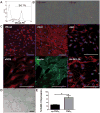
Approach and results: We validated and optimized an adherent, feeder-free differentiation procedure to derive early vascular cells (EVCs) with high portions of vascular endothelial cadherin-positive cells from hiPSCs. We demonstrate similar differentiation efficiency from hiPSCs derived from healthy donor and patients with T1D. T1D-hiPSC-derived vascular endothelial cadherin-positive cells can mature to functional endothelial cells-expressing mature markers: von Willebrand factor and endothelial nitric oxide synthase are capable of lectin binding and acetylated low-density lipoprotein uptake, form cords in Matrigel and respond to tumor necrosis factor-α. When embedded in engineered hyaluronic acid hydrogels, T1D-EVCs undergo morphogenesis and assemble into 3D networks. When encapsulated in a novel hypoxia-inducible hydrogel, T1D-EVCs respond to low oxygen and form 3D networks. As xenografts, T1D-EVCs incorporate into developing zebrafish vasculature.
Conclusions: Using our robust protocol, we can direct efficient differentiation of T1D-hiPSC to EVCs. Early endothelial cells derived from T1D-hiPSC are functional when mature. T1D-EVCs self-assembled into 3D networks when embedded in hyaluronic acid and hypoxia-inducible hydrogels. The capability of T1D-EVCs to assemble into 3D networks in engineered matrices and to respond to a hypoxic microenvironment is a significant advancement for autologous vascular therapy in diabetic patients and has broad importance for tissue engineering.
Keywords: diabetes mellitus; endothelial cells; hydrogels; induced pluripotent stem cells; vascular networks.
© 2015 American Heart Association, Inc.
Figures



References
-
- Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2010. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;61:1–117. - PubMed
-
- Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annual review of medicine. 2002;53:245–267. - PubMed
-
- Wilson PW. Diabetes mellitus and coronary heart disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1998;32:S89–100. - PubMed
-
- Simons M. Angiogenesis, arteriogenesis, and diabetes: Paradigm reassessed? Journal of the American College of Cardiology. 2005;46:835–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

