Detection of Typhoidal and Paratyphoidal Salmonella in Blood by Real-time Polymerase Chain Reaction
- PMID: 26449938
- PMCID: PMC6279176
- DOI: 10.1093/cid/civ726
Detection of Typhoidal and Paratyphoidal Salmonella in Blood by Real-time Polymerase Chain Reaction
Abstract
Background: The gold standard for diagnosis of enteric fever caused by Salmonella Typhi or Salmonella Paratyphi A or B is bone marrow culture. However, because bone marrow aspiration is highly invasive, many hospitals and large health centers perform blood culture instead. As blood culture has several limitations, there is a need for novel typhoid diagnostics with improved sensitivity and more rapid time to detection.
Methods: We developed a clyA-based real-time polymerase chain reaction (qPCR) method to detect Salmonella Typhi and Salmonella Paratyphi A simultaneously in blood. The sensitivity and specificity of this probeset was first evaluated in vitro in the laboratory and then in a typhoid-endemic population, in Karachi, Pakistan, and in healthy US volunteers.
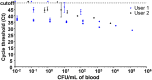
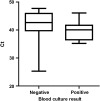
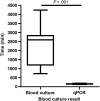
Results: We optimized a DNA extraction and real-time PCR-based method that could reliably detect 1 colony-forming unit/mL of Salmonella Typhi. The probe set was able to detect clinical Salmonella Typhi and Salmonella Paratyphi A strains and also diarrheagenic Escherichia coli, but not invasive E. coli or other invasive bacteria. In the field, the clyA qPCR diagnostic was 40% as sensitive as blood culture. However, when qPCR-positive specimens were considered to be true positives, blood culture only exhibited 28.57% sensitivity. Specificity was ≥90% for all comparisons and in the healthy US volunteers. qPCR was significantly faster than blood culture in terms of detection of typhoid and paratyphoid.
Conclusions: Based on lessons learned, we recommend that future field trials of this and other novel diagnostics that detect typhoidal and nontyphoidal Salmonella employ multiple methodologies to define a "positive" sample.
Keywords: PCR; Salmonella; blood; diagnostic; typhoid.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Clinical Evaluation of a Multiplex PCR for the Detection of Salmonella enterica Serovars Typhi and Paratyphi A from Blood Specimens in a High-Endemic Setting.Am J Trop Med Hyg. 2019 Sep;101(3):513-520. doi: 10.4269/ajtmh.18-0992. Am J Trop Med Hyg. 2019. PMID: 31287048 Free PMC article.
-
PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B among Salmonella Isolates from the blood of patients with clinical enteric fever.J Clin Microbiol. 2008 May;46(5):1861-6. doi: 10.1128/JCM.00109-08. Epub 2008 Mar 26. J Clin Microbiol. 2008. PMID: 18367574 Free PMC article.
-
Multiplex PCR for differential diagnosis of emerging typhoidal pathogens directly from blood samples.Epidemiol Infect. 2009 Jan;137(1):102-7. doi: 10.1017/S0950268808000654. Epub 2008 Apr 16. Epidemiol Infect. 2009. PMID: 18413005
-
Pancreatitis in enteric fever.Indian J Gastroenterol. 2002 Jan-Feb;21(1):32-3. Indian J Gastroenterol. 2002. PMID: 11871836 Review.
-
Comparative accuracy of typhoid diagnostic tools: A Bayesian latent-class network analysis.PLoS Negl Trop Dis. 2019 May 8;13(5):e0007303. doi: 10.1371/journal.pntd.0007303. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31067228 Free PMC article.
Cited by
-
Typhoid fever.Nat Rev Dis Primers. 2023 Dec 14;9(1):71. doi: 10.1038/s41572-023-00480-z. Nat Rev Dis Primers. 2023. PMID: 38097589 Review.
-
Clinical Evaluation of a Multiplex PCR for the Detection of Salmonella enterica Serovars Typhi and Paratyphi A from Blood Specimens in a High-Endemic Setting.Am J Trop Med Hyg. 2019 Sep;101(3):513-520. doi: 10.4269/ajtmh.18-0992. Am J Trop Med Hyg. 2019. PMID: 31287048 Free PMC article.
-
Detecting Residual Chronic Salmonella Typhi Carriers on the Road to Typhoid Elimination in Santiago, Chile, 2017-2019.J Infect Dis. 2024 Aug 16;230(2):e254-e267. doi: 10.1093/infdis/jiad585. J Infect Dis. 2024. PMID: 38123455 Free PMC article.
-
Fever in the Returned Pediatric Traveler.Glob Pediatr Health. 2021 Aug 16;8:2333794X211026188. doi: 10.1177/2333794X211026188. eCollection 2021. Glob Pediatr Health. 2021. PMID: 34423077 Free PMC article. Review.
-
Classification of Salmonella enterica of the (Para-)Typhoid Fever Group by Fourier-Transform Infrared (FTIR) Spectroscopy.Microorganisms. 2021 Apr 15;9(4):853. doi: 10.3390/microorganisms9040853. Microorganisms. 2021. PMID: 33921159 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

