Role of the renal sympathetic nerve in renal glucose metabolism during the development of type 2 diabetes in rats
- PMID: 26450431
- PMCID: PMC4630257
- DOI: 10.1007/s00125-015-3771-9
Role of the renal sympathetic nerve in renal glucose metabolism during the development of type 2 diabetes in rats
Abstract
Aims/hypothesis: Recent clinical studies have shown that renal sympathetic denervation (RDX) improves glucose metabolism in patients with resistant hypertension. We aimed to elucidate the potential contribution of the renal sympathetic nervous system to glucose metabolism during the development of type 2 diabetes.
Methods: Uninephrectomised diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats underwent RDX at 25 weeks of age and were followed up to 46 weeks of age.
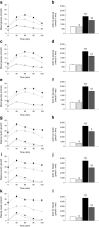
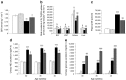
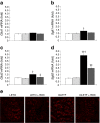
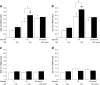
Results: RDX decreased plasma and renal tissue noradrenaline (norepinephrine) levels and BP. RDX also improved glucose metabolism and insulin sensitivity, which was associated with increased in vivo glucose uptake by peripheral tissues. Furthermore, RDX suppressed overexpression of sodium-glucose cotransporter 2 (Sglt2 [also known as Slc5a2]) in renal tissues, which was followed by an augmentation of glycosuria in type 2 diabetic OLETF rats. Similar improvements in glucose metabolism after RDX were observed in young OLETF rats at the prediabetic stage (21 weeks of age) without changing BP.
Conclusions/interpretation: Here, we propose the new concept of a connection between renal glucose metabolism and the renal sympathetic nervous system during the development of type 2 diabetes. Our data demonstrate that RDX exerts beneficial effects on glucose metabolism by an increase in tissue glucose uptake and glycosuria induced by Sglt2 suppression. These data have provided a new insight not only into the treatment of hypertensive type 2 diabetic patients, but also the pathophysiology of insulin resistance manifested by sympathetic hyperactivity.
Keywords: Blood pressure; Glucose metabolism; Insulin resistance; Renal sympathetic denervation (RDX); Sglt2; Slc5a2; Sodium-glucose cotransporter 2; Type 2 diabetes.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

