Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer
- PMID: 26450505
- PMCID: PMC4718738
- DOI: 10.1007/s10549-015-3590-1
Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer
Abstract
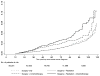
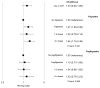
Risk of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) post-breast cancer treatment with adjuvant chemotherapy and granulocyte colony-stimulating factors (G-CSF) is not fully characterized. Our objective was to estimate MDS/AML risk associated with specific breast cancer treatments. We conducted a retrospective cohort study of women aged ≥66 years with stage I-III breast cancer between 2001 and 2009 using the Surveillance, Epidemiology, and End Results-Medicare database. Women were classified as receiving treatment with radiation, chemotherapy, and/or G-CSF. We used multivariable Cox proportional hazards models to estimate adjusted hazard ratios (HR) and 95 % confidence intervals (CI) for MDS/AML risk. Among 56,251 breast cancer cases, 1.2 % developed MDS/AML during median follow-up of 3.2 years. 47.1 % of women received radiation and 14.3 % received chemotherapy. Compared to breast cancer cases treated with surgery alone, those treated with chemotherapy (HR = 1.38, 95 %-CI 0.98-1.93) and chemotherapy/radiation (HR = 1.77, 95 %-CI 1.25-2.51) had increased risk of MDS/AML, but not radiation alone (HR = 1.08, 95 % CI 0.86-1.36). Among chemotherapy regimens and G-CSF, MDS/AML risk was differentially associated with anthracycline/cyclophosphamide-containing regimens (HR = 1.86, 95 %-CI 1.33-2.61) and filgrastim (HR = 1.47, 95 %-CI 1.05-2.06), but not pegfilgrastim (HR = 1.10, 95 %-CI 0.73-1.66). We observed increased MDS/AML risk among older breast cancer survivors treated with anthracycline/cyclophosphamide chemotherapy that was enhanced by G-CSF. Although small, this risk warrants consideration when determining adjuvant chemotherapy and neutropenia prophylaxis for breast cancer patients.
Keywords: Acute myeloid leukemia; Adjuvant chemotherapy; Breast cancer; Granulocyte colony-stimulating factors; Myelodysplastic syndrome.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures

References
-
- Wolff AC, Blackford AL, Visvanathan K, Rugo HS, Moy B, Goldstein LJ, Stockerl-Goldstein K, Neumayer L, Langbaum TS, Theriault RL, Hughes ME, Weeks JC, Karp JE. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33(4):340–348. doi: 10.1200/JCO.2013.54.6119. - DOI - PMC - PubMed
-
- Praga C, Bergh J, Bliss J, Bonneterre J, Cesana B, Coombes RC, Fargeot P, Folin A, Fumoleau P, Giuliani R, Kerbrat P, Hery M, Nilsson J, Onida F, Piccart M, Shepherd L, Therasse P, Wils J, Rogers D. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005;23(18):4179–4191. doi: 10.1200/JCO.2005.05.029. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

