Wafer-scale growth of VO2 thin films using a combinatorial approach
- PMID: 26450653
- PMCID: PMC4633718
- DOI: 10.1038/ncomms9475
Wafer-scale growth of VO2 thin films using a combinatorial approach
Abstract
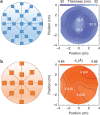
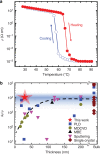
Transition metal oxides offer functional properties beyond conventional semiconductors. Bridging the gap between the fundamental research frontier in oxide electronics and their realization in commercial devices demands a wafer-scale growth approach for high-quality transition metal oxide thin films. Such a method requires excellent control over the transition metal valence state to avoid performance deterioration, which has been proved challenging. Here we present a scalable growth approach that enables a precise valence state control. By creating an oxygen activity gradient across the wafer, a continuous valence state library is established to directly identify the optimal growth condition. Single-crystalline VO2 thin films have been grown on wafer scale, exhibiting more than four orders of magnitude change in resistivity across the metal-to-insulator transition. It is demonstrated that 'electronic grade' transition metal oxide films can be realized on a large scale using a combinatorial growth approach, which can be extended to other multivalent oxide systems.
Figures




References
-
- Jany R. et al. Monolithically integrated circuits from functional oxides. Adv. Mater. Interfaces 1, 1–7 (2014).
-
- Mathews S., Ramesh R., Venkatesan T. & Benedetto J. Ferroelectric field effect transistor based on epitaxial perovskite heterostructures. Science 276, 238–240 (1997). - PubMed
-
- Takagi H. & Hwang H. Y. An emergent change of phase for electronics. Science 327, 1601–1602 (2010). - PubMed
-
- Assmann E. et al. Oxide heterostructures for efficient solar cells. Phys. Rev. Lett. 110, 078701 (2013). - PubMed
-
- Katzke H., Tolédano P. & Depmeier W. Theory of morphotropic transformations in vanadium oxides. Phys. Rev. B 68, 024109 (2003).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

