Direct Evidence for Microdomain-Specific Localization and Remodeling of Functional L-Type Calcium Channels in Rat and Human Atrial Myocytes
- PMID: 26450916
- PMCID: PMC4689179
- DOI: 10.1161/CIRCULATIONAHA.115.018131
Direct Evidence for Microdomain-Specific Localization and Remodeling of Functional L-Type Calcium Channels in Rat and Human Atrial Myocytes
Abstract
Background: Distinct subpopulations of L-type calcium channels (LTCCs) with different functional properties exist in cardiomyocytes. Disruption of cellular structure may affect LTCC in a microdomain-specific manner and contribute to the pathophysiology of cardiac diseases, especially in cells lacking organized transverse tubules (T-tubules) such as atrial myocytes (AMs).
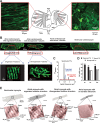
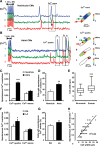
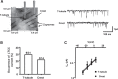
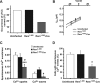
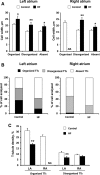
Methods and results: Isolated rat and human AMs were characterized by scanning ion conductance, confocal, and electron microscopy. Half of AMs possessed T-tubules and structured topography, proportional to cell width. A bigger proportion of myocytes in the left atrium had organized T-tubules and topography than in the right atrium. Super-resolution scanning patch clamp showed that LTCCs distribute equally in T-tubules and crest areas of the sarcolemma, whereas, in ventricular myocytes, LTCCs primarily cluster in T-tubules. Rat, but not human, T-tubule LTCCs had open probability similar to crest LTCCs, but exhibited ≈ 40% greater current. Optical mapping of Ca(2+) transients revealed that rat AMs presented ≈ 3-fold as many spontaneous Ca(2+) release events as ventricular myocytes. Occurrence of crest LTCCs and spontaneous Ca(2+) transients were eliminated by either a caveolae-targeted LTCC antagonist or disrupting caveolae with methyl-β-cyclodextrin, with an associated ≈ 30% whole-cell ICa,L reduction. Heart failure (16 weeks post-myocardial infarction) in rats resulted in a T-tubule degradation (by ≈ 40%) and significant elevation of spontaneous Ca(2+) release events. Although heart failure did not affect LTCC occurrence, it led to ≈ 25% decrease in T-tubule LTCC amplitude.
Conclusions: We provide the first direct evidence for the existence of 2 distinct subpopulations of functional LTCCs in rat and human AMs, with their biophysical properties modulated in heart failure in a microdomain-specific manner.
Keywords: T-tubules; calcium channels; heart atria; heart failure; myocytes, cardiac; scanning ion conductance microscopy.
© 2015 The Authors.
Figures



References
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. - PubMed
-
- Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol Heart Circ Physiol. 2002;283:H1720–H1728. doi: 10.1152/ajpheart.00347.2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

