Chitosan nanofiber scaffold improves bone healing via stimulating trabecular bone production due to upregulation of the Runx2/osteocalcin/alkaline phosphatase signaling pathway
- PMID: 26451104
- PMCID: PMC4590342
- DOI: 10.2147/IJN.S90669
Chitosan nanofiber scaffold improves bone healing via stimulating trabecular bone production due to upregulation of the Runx2/osteocalcin/alkaline phosphatase signaling pathway
Abstract
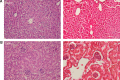
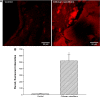
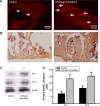
Osteoblasts play critical roles in bone formation. Our previous study showed that chitosan nanofibers can stimulate osteoblast proliferation and maturation. This translational study used an animal model of bone defects to evaluate the effects of chitosan nanofiber scaffolds on bone healing and the possible mechanisms. In this study, we produced uniform chitosan nanofibers with fiber diameters of approximately 200 nm. A bone defect was surgically created in the proximal femurs of male C57LB/6 mice, and then the left femur was implanted with chitosan nanofiber scaffolds for 21 days and compared with the right femur, which served as a control. Histological analyses revealed that implantation of chitosan nanofiber scaffolds did not lead to hepatotoxicity or nephrotoxicity. Instead, imaging analyses by X-ray transmission and microcomputed tomography showed that implantation of chitosan nanofiber scaffolds improved bone healing compared with the control group. In parallel, microcomputed tomography and bone histomorphometric assays further demonstrated augmentation of the production of new trabecular bone in the chitosan nanofiber-treated group. Furthermore, implantation of chitosan nanofiber scaffolds led to a significant increase in the trabecular bone thickness but a reduction in the trabecular parameter factor. As to the mechanisms, analysis by confocal microscopy showed that implantation of chitosan nanofiber scaffolds increased levels of Runt-related transcription factor 2 (Runx2), a key transcription factor that regulates osteogenesis, in the bone defect sites. Successively, amounts of alkaline phosphatase and osteocalcin, two typical biomarkers that can simulate bone maturation, were augmented following implantation of chitosan nanofiber scaffolds. Taken together, this translational study showed a beneficial effect of chitosan nanofiber scaffolds on bone healing through stimulating trabecular bone production due to upregulation of Runx2-mediated alkaline phosphatase and osteocalcin gene expressions. Our results suggest the potential of chitosan nanofiber scaffolds for therapy of bone diseases, including bone defects and bone fractures.
Keywords: Runx2/OCN/ALP; bone healing; bone histomorphometry; chitosan nanofibers; micro-computed tomography.
Figures





Similar articles
-
Improving effects of chitosan nanofiber scaffolds on osteoblast proliferation and maturation.Int J Nanomedicine. 2014 Sep 9;9:4293-304. doi: 10.2147/IJN.S68012. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25246786 Free PMC article.
-
Genistein Improves Bone Healing via Triggering Estrogen Receptor Alpha-Mediated Expressions of Osteogenesis-Associated Genes and Consequent Maturation of Osteoblasts.J Agric Food Chem. 2020 Sep 30;68(39):10639-10650. doi: 10.1021/acs.jafc.0c02830. Epub 2020 Sep 8. J Agric Food Chem. 2020. PMID: 32897066
-
Inhibition of the estrogen receptor alpha signaling delays bone regeneration and alters osteoblast maturation, energy metabolism, and angiogenesis.Life Sci. 2020 Oct 1;258:118195. doi: 10.1016/j.lfs.2020.118195. Epub 2020 Aug 8. Life Sci. 2020. PMID: 32781073
-
The Application of microRNAs in Biomaterial Scaffold-Based Therapies for Bone Tissue Engineering.Biotechnol J. 2019 Oct;14(10):e1900084. doi: 10.1002/biot.201900084. Epub 2019 Jul 9. Biotechnol J. 2019. PMID: 31166084 Review.
-
Chitin and chitosan nanofibers: preparation and chemical modifications.Molecules. 2014 Nov 11;19(11):18367-80. doi: 10.3390/molecules191118367. Molecules. 2014. PMID: 25393598 Free PMC article. Review.
Cited by
-
Mineralization in a Critical Size Bone-Gap in Sheep Tibia Improved by a Chitosan-Calcium Phosphate-Based Composite as Compared to Predicate Device.Materials (Basel). 2022 Jan 22;15(3):838. doi: 10.3390/ma15030838. Materials (Basel). 2022. PMID: 35160784 Free PMC article.
-
The Bradykinin-BDKRB1 Axis Regulates Aquaporin 4 Gene Expression and Consequential Migration and Invasion of Malignant Glioblastoma Cells via a Ca2+-MEK1-ERK1/2-NF-κB Mechanism.Cancers (Basel). 2020 Mar 13;12(3):667. doi: 10.3390/cancers12030667. Cancers (Basel). 2020. PMID: 32182968 Free PMC article.
-
CYT387, a JAK-Specific Inhibitor Impedes Osteoclast Activity and Oophorectomy-Induced Osteoporosis via Modulating RANKL and ROS Signaling Pathways.Front Pharmacol. 2022 Mar 8;13:829862. doi: 10.3389/fphar.2022.829862. eCollection 2022. Front Pharmacol. 2022. PMID: 35345816 Free PMC article.
-
Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects.Front Bioeng Biotechnol. 2022 May 4;10:899760. doi: 10.3389/fbioe.2022.899760. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35600891 Free PMC article. Review.
-
Crosstalk between chitosan and cell signaling pathways.Cell Mol Life Sci. 2019 Jul;76(14):2697-2718. doi: 10.1007/s00018-019-03107-3. Epub 2019 Apr 27. Cell Mol Life Sci. 2019. PMID: 31030227 Free PMC article. Review.
References
-
- Seeman E, Delmas PD. Bone quality – the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. - PubMed
-
- Karsdal MA, Martin TJ, Henriksen K. Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int. 2014;94:88–97. - PubMed
-
- Cauley JA, Chalhoub D, Kassem AM, Fuleihan Gel-H. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol. 2014;10:338–351. - PubMed
-
- Geris L, Gerisch A, Sloten JV, Weiner R, Oosterwyck HV. Angiogenesis in bone fracture healing: a bioregulatory model. J Theor Biol. 2008;251:137–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

