Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia
- PMID: 26451310
- PMCID: PMC4590028
- DOI: 10.1080/2162402X.2015.1027469
Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia
Abstract
The engineering of T lymphocytes to express chimeric antigen receptors (CARs) aims to establish T cell-mediated tumor immunity rapidly. In this study, we conducted a pilot clinical trial of autologous or donor- derived T cells genetically modified to express a CAR targeting the B-cell antigen CD19 harboring 4-1BB and the CD3ζ moiety. All enrolled patients had relapsed or chemotherapy-refractory B-cell lineage acute lymphocytic leukemia (B-ALL). Of the nine patients, six had definite extramedullary involvement, and the rate of overall survival at 18 weeks was 56%. One of the two patients who received conditioning chemotherapy achieved a three-month durable complete response with partial regression of extramedullary lesions. Four of seven patients who did not receive conditioning chemotherapy achieved dramatic regression or a mixed response in the haematopoietic system and extramedullary tissues for two to nine months. Grade 2-3 graft-versus-host disease (GVHD) was observed in two patients who received substantial donor-derived anti-CD19 CART (chimeric antigen receptor-modified T) cells 3-4 weeks after cell infusions. These results show for the first time that donor-derived anti-CD19 CART cells can cause GVHD and regression of extramedullary B-ALL. This study is registered at www.clinicaltrials.gov as NCT01864889.
Keywords: B-cell acute lymphoblastic leukemia (B-ALL); anti-CD19 chimeric antigen receptor (CAR) T cells; graft-versus-host disease (GVHD); refractory.
Figures

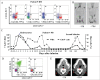
 indicates the time of relapse, ↑
indicates the time of relapse, ↑ indicates the time of second infusion, ↓
indicates the time of second infusion, ↓ indicates the time of chemotherapy, black squares represent the values for CAR copies by Q-PCR, and black circles indicate CD19+ B cell counts in PB. The first chemotherapy regimen: Cyclophosphamide Etoposide, Vincristine and Dexamethasone. The second chemotherapy regimen: Vincristine, Daunorubicin, Cyclophosphamide and Prednison (B, C) For patient Bone marrow and cerebralspinal fluid aspirates were obtained at serial time points after CART-19 cell infusion in patient 7. Black squares represent the values of CAR copies by Q-PCR and black circles indicate the detection of bcr/abl transcripts.
indicates the time of chemotherapy, black squares represent the values for CAR copies by Q-PCR, and black circles indicate CD19+ B cell counts in PB. The first chemotherapy regimen: Cyclophosphamide Etoposide, Vincristine and Dexamethasone. The second chemotherapy regimen: Vincristine, Daunorubicin, Cyclophosphamide and Prednison (B, C) For patient Bone marrow and cerebralspinal fluid aspirates were obtained at serial time points after CART-19 cell infusion in patient 7. Black squares represent the values of CAR copies by Q-PCR and black circles indicate the detection of bcr/abl transcripts.
 indicates the time of second infusion and ↓
indicates the time of second infusion and ↓ indicates hydroxyurea injection. (D) Flow cytometry for CD20 and CD19 expression in PB before and after treatment. Cells were gated on CD45+7AAD− cells in patient 4. (E) A CT scan shows regression of cervical lymph nodes in patient 4 after infusion of CART-19 cells. ↑
indicates hydroxyurea injection. (D) Flow cytometry for CD20 and CD19 expression in PB before and after treatment. Cells were gated on CD45+7AAD− cells in patient 4. (E) A CT scan shows regression of cervical lymph nodes in patient 4 after infusion of CART-19 cells. ↑ indicates a lymph node mass that regressed.
indicates a lymph node mass that regressed.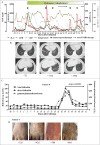
 indicates the bronchiectasis-like imaging features or ground-glass changes. (C) This panel shows changes in the levels of total bilirubin, direct bilirubin and indirect bilirubin during the period of in which patient 8 developed GVHD. (D) This panel shows chronically aggravated skin damage in patient 9 due to GVHD.
indicates the bronchiectasis-like imaging features or ground-glass changes. (C) This panel shows changes in the levels of total bilirubin, direct bilirubin and indirect bilirubin during the period of in which patient 8 developed GVHD. (D) This panel shows chronically aggravated skin damage in patient 9 due to GVHD.References
-
- Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A, Kobbe G, Kreuzer KA, Leimer L, Reichle A et al.. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120:2032-41; PMID:22493293; http://dx.doi.org/10.1182/blood-2011-12-399287 - DOI - PubMed
-
- Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood 2013; 121:1077-82; PMID:23243288; http://dx.doi.org/10.1182/blood-2012-08-234492 - DOI - PMC - PubMed
-
- Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, Sandlund JT, Hudson M, Relling M, Evans WE et al.. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer 2005; 103:368-76; PMID:15599932; http://dx.doi.org/10.1002/cncr.20743 - DOI - PubMed
-
- Gajjar A, Harrison PL, Sandlund JT, Rivera GK, Ribeiro RC, Rubnitz JE, Razzouk B, Relling MV, Evans WE, Boyett JM et al.. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood 2000; 96:3381-4; PMID:11071631 - PubMed
-
- Sandlund JT, Harrison PL, Rivera G, Behm FG, Head D, Boyett J, Rubnitz JE, Gajjar A, Raimondi S, Ribeiro R et al.. Persistence of lymphoblasts in bone marrow on day 15 and days 22 to 25 of remission induction predicts a dismal treatment outcome in children with acute lymphoblastic leukemia. Blood 2002; 100:43-7; PMID:12070006; http://dx.doi.org/10.1182/blood.V100.1.43 - DOI - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
