Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity
- PMID: 26451485
- PMCID: PMC4826740
- DOI: 10.1016/j.cell.2015.08.030
Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity
Abstract
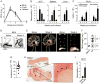
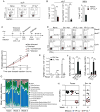
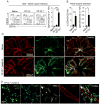
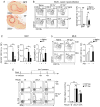
Infections have been proposed as initiating factors for inflammatory disorders; however, identifying associations between defined infectious agents and the initiation of chronic disease has remained elusive. Here, we report that a single acute infection can have dramatic and long-term consequences for tissue-specific immunity. Following clearance of Yersinia pseudotuberculosis, sustained inflammation and associated lymphatic leakage in the mesenteric adipose tissue deviates migratory dendritic cells to the adipose compartment, thereby preventing their accumulation in the mesenteric lymph node. As a consequence, canonical mucosal immune functions, including tolerance and protective immunity, are persistently compromised. Post-resolution of infection, signals derived from the microbiota maintain inflammatory mesentery remodeling and consequently, transient ablation of the microbiota restores mucosal immunity. Our results indicate that persistent disruption of communication between tissues and the immune system following clearance of an acute infection represents an inflection point beyond which tissue homeostasis and immunity is compromised for the long-term. VIDEO ABSTRACT.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Comment in
-
IMMUNOLOGY. From transient infection to chronic disease.Science. 2015 Oct 9;350(6257):161. doi: 10.1126/science.aad4141. Science. 2015. PMID: 26450196 No abstract available.
-
Winning the Microbial Battle, but Not the War.Cell. 2015 Oct 8;163(2):271-2. doi: 10.1016/j.cell.2015.09.050. Cell. 2015. PMID: 26451475
-
The Infectious Cause of the Chronic Effect.Cell Host Microbe. 2015 Oct 14;18(4):383-5. doi: 10.1016/j.chom.2015.09.011. Cell Host Microbe. 2015. PMID: 26468738
-
Immunology: Chronic effects of acute infections.Nature. 2015 Oct 22;526(7574):509-10. doi: 10.1038/526509a. Nature. 2015. PMID: 26490613 No abstract available.
-
Mucosal immunology: Battle scars.Nat Rev Immunol. 2015 Nov;15(11):668. doi: 10.1038/nri3932. Nat Rev Immunol. 2015. PMID: 26494049 No abstract available.
References
-
- Asano S. Granulomatous lymphadenitis. J Clin Exp Hematop. 2012;52:1–16. - PubMed
-
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. - PubMed
-
- Behr MA. The path to Crohn's disease: is mucosal pathology a secondary event? Inflamm Bowel Dis. 2010;16:896–902. - PubMed
-
- Bekiaris V, Persson EK, Agace WW. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol Rev. 2014;260:86–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

