The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation
- PMID: 26451609
- PMCID: PMC4742005
- DOI: 10.18632/oncotarget.5256
The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation
Abstract
Neoadjuvant chemoradiotherapy (nCRT) followed by surgery is the mainstay treatment for locally advanced rectal cancer. Variable degrees of tumor regression are observed after nCRT and alternative treatment strategies, including close surveillance without immediate surgery, have been investigated to spare patients with complete tumor regression from potentially adverse outcomes of radical surgery. However, clinical and radiological assessment of response does not allow accurate identification of patients with complete response. In addition, surveillance for recurrence is similarly important for these patients, as early detection of recurrence allows salvage resections and adjuvant interventions. We report the use of liquid biopsies and personalized biomarkers for monitoring treatment response to nCRT and detecting residual disease and recurrence in patients with rectal cancer. We sequenced the whole-genome of four rectal tumors to identify patient-specific chromosomal rearrangements that were used to monitor circulating tumor DNA (ctDNA) in liquid biopsies collected at diagnosis and during nCRT and follow-up. We compared ctDNA levels to clinical, radiological and pathological response to nCRT. Our results indicate that personalized biomarkers and liquid biopsies may not be sensitive for the detection of microscopic residual disease. However, it can be efficiently used to monitor treatment response to nCRT and detect disease recurrence, preceding increases in CEA levels and radiological diagnosis. Similar good results were observed when assessing tumor response to systemic therapy and disease progression. Our study supports the use of personalized biomarkers and liquid biopsies to tailor the management of rectal cancer patients, however, replication in a larger cohort is necessary to introduce this strategy into clinical practice.
Keywords: ctDNA; liquid biopsies; neoadjuvant therapy; personalized biomarkers; rectal cancer.
Conflict of interest statement
No, there is no conflict of interest.
Figures

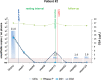


References
-
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rodel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012;30:1926–1933. - PubMed
-
- Sanghera P, Wong DWY, McConkey CC, Geh JI, Hartley JI. Chemoradiotherapy for Rectal Cancer: An Updated Analysis of Factors Affecting Pathological Response. Clin. Oncol. 2008;20:176–183. - PubMed
-
- Habr-Gama A, Perez RO, São Julião GP, Proscurshim I, Gama-Rodrigues J. Nonoperative approaches to rectal cancer: a critical evaluation. Semin. Radiat. Oncol. 2011;21:234–239. - PubMed
-
- Smith FM, Rao C, Oliva Perez R, Bujko K, Athanasiou T, Habr-Gama A, Faiz O. Avoiding Radical Surgery Improves Early Survival in Elderly Patients With Rectal Cancer, Demonstrating Complete Clinical Response After Neoadjuvant Therapy. Dis. Colon Rectum. 2015;58:159–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

