Efficacy and Safety of Umeclidinium Added to Fluticasone Propionate/Salmeterol in Patients with COPD: Results of Two Randomized, Double-Blind Studies
- PMID: 26451734
- PMCID: PMC4778542
- DOI: 10.3109/15412555.2015.1034256
Efficacy and Safety of Umeclidinium Added to Fluticasone Propionate/Salmeterol in Patients with COPD: Results of Two Randomized, Double-Blind Studies
Abstract
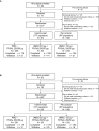
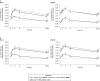
Combinations of drugs with distinct and complementary mechanisms of action may offer improved efficacy in the treatment of chronic obstructive pulmonary disease (COPD). In two 12-week, double-blind, parallel-group studies, patients with COPD were randomized 1:1:1 to once-daily umeclidinium (UMEC; 62.5 μg and 125 μg) or placebo (PBO), added to twice-daily fluticasone propionate/salmeterol (FP/SAL; 250/50 μg). In both studies, the primary efficacy measure was trough forced expiratory volume in 1 second (FEV1) at Day 85. Secondary endpoints were weighted-mean (WM) FEV1 over 0-6 hours post-dose (Day 84) and rescue albuterol use. Health-related quality of life outcomes (St. George's Respiratory Questionnaire [SGRQ] and COPD assessment test [CAT]) were also examined. Safety was assessed throughout. Both UMEC+FP/SAL doses provided statistically significant improvements in trough FEV1 (Day 85: 0.127-0.148 L) versus PBO+FP/SAL. Similarly, both UMEC+FP/SAL doses provided statistically-significant improvements in 0-6 hours post-dose WM FEV1 versus PBO+FP/SAL (Day 84: 0.144-0.165 L). Rescue use over Weeks 1-12 decreased with UMEC+FP/SAL in both studies versus PBO+FP/SAL (Study 1, 0.3 puffs/day [both doses]; Study 2, 0.5 puffs/day [UMEC 125+FP/SAL]). Decreases from baseline in CAT score were generally larger for both doses of UMEC+FP/SAL versus PBO+FP/SAL (except for Day 84 Study 2). In Study 1, no differences in SGRQ score were observed between UMEC+FP/SAL and PBO+FP/SAL; however, in Study 2, statistically significant improvements were observed with UMEC 62.5+FP/SAL (Day 28) and UMEC 125+FP/SAL (Days 28 and 84) versus PBO+FP/SAL. The incidence of on-treatment adverse events across all treatment groups was 37-41% in Study 1 and 36-38% in Study 2. Overall, these data indicate that the combination of UMEC+FP/SAL can provide additional benefits over FP/SAL alone in patients with COPD.
Keywords: bronchodilation; inhaled corticosteroid; long-acting beta agonist; long-acting muscarinic antagonist.
Figures


References
-
- Brusasco V. Reducing cholinergic constriction: the major reversible mechanism in COPD. Eur Respir Rev 2006. 15:32–36.
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014 Available from: http://www.goldcopd.org/ accessed July 31 2014. - PubMed
-
- Singh D, Brooks J, Hagan G, et al. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63:592–598. - PubMed
-
- Cazzola M, Ando F, Santus P, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther. 2007;20:556–561. - PubMed
-
- Hanania NA, Crater GD, Morris AN, et al. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106:91–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
