Receptor-Interacting Protein Kinase 3 Deficiency Delays Cutaneous Wound Healing
- PMID: 26451737
- PMCID: PMC4599740
- DOI: 10.1371/journal.pone.0140514
Receptor-Interacting Protein Kinase 3 Deficiency Delays Cutaneous Wound Healing
Abstract
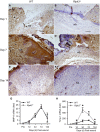
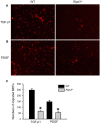
Wound healing consists of a complex, dynamic and overlapping process involving inflammation, proliferation and tissue remodeling. A better understanding of wound healing process at the molecular level is needed for the development of novel therapeutic strategies. Receptor-interacting protein kinase 3 (RIPK3) controls programmed necrosis in response to TNF-α during inflammation and has been shown to be highly induced during cutaneous wound repair. However, its role in wound healing remains to be demonstrated. To study this, we created dorsal cutaneous wounds on male wild-type (WT) and RIPK3-deficient (Ripk3-/-) mice. Wound area was measured daily until day 14 post-wound and skin tissues were collected from wound sites at various days for analysis. The wound healing rate in Ripk3-/- mice was slower than the WT mice over the 14-day course; especially, at day 7, the wound size in Ripk3-/- mice was 53% larger than that of WT mice. H&E and Masson-Trichrome staining analysis showed impaired quality of wound closure in Ripk3-/- wounds with delayed re-epithelialization and angiogenesis and defected granulation tissue formation and collagen deposition compared to WT. The neutrophil infiltration pattern was altered in Ripk3-/- wounds with less neutrophils at day 1 and more neutrophils at day 3. This altered pattern was also reflected in the differential expression of IL-6, KC, IL-1β and TNF-α between WT and Ripk3-/- wounds. MMP-9 protein expression was decreased with increased Timp-1 mRNA in the Ripk3-/- wounds compared to WT. The microvascular density along with the intensity and timing of induction of proangiogenic growth factors VEGF and TGF-β1 were also decreased or delayed in the Ripk3-/- wounds. Furthermore, mouse embryonic fibroblasts (MEFs) from Ripk3-/- mice migrated less towards chemoattractants TGF-β1 and PDGF than MEFs from WT mice. These results clearly demonstrate that RIPK3 is an essential molecule to maintain the temporal manner of the normal progression of wound closure.
Conflict of interest statement
Figures





Similar articles
-
A deficiency in cold-inducible RNA-binding protein accelerates the inflammation phase and improves wound healing.Int J Mol Med. 2016 Feb;37(2):423-8. doi: 10.3892/ijmm.2016.2451. Epub 2016 Jan 7. Int J Mol Med. 2016. PMID: 26743936
-
Rotational stress-induced increase in epinephrine levels delays cutaneous wound healing in mice.Brain Behav Immun. 2010 Mar;24(3):427-37. doi: 10.1016/j.bbi.2009.11.012. Epub 2009 Nov 26. Brain Behav Immun. 2010. PMID: 19944145
-
Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration.FASEB J. 2002 Jul;16(9):963-74. doi: 10.1096/fj.01-0776com. FASEB J. 2002. PMID: 12087057
-
[The modern approach to wound treatment].Med Pregl. 2000 Jul-Aug;53(7-8):363-8. Med Pregl. 2000. PMID: 11214479 Review. Croatian.
-
The role of nuclear hormone receptors in cutaneous wound repair.Cell Biochem Funct. 2015 Jan;33(1):1-13. doi: 10.1002/cbf.3086. Epub 2014 Dec 22. Cell Biochem Funct. 2015. PMID: 25529612 Free PMC article. Review.
Cited by
-
Experimental models and methods for cutaneous wound healing assessment.Int J Exp Pathol. 2020 Feb;101(1-2):21-37. doi: 10.1111/iep.12346. Epub 2020 Mar 30. Int J Exp Pathol. 2020. PMID: 32227524 Free PMC article. Review.
-
RIPK1/RIPK3 promotes vascular permeability to allow tumor cell extravasation independent of its necroptotic function.Cell Death Dis. 2017 Feb 2;8(2):e2588. doi: 10.1038/cddis.2017.20. Cell Death Dis. 2017. PMID: 28151480 Free PMC article.
-
Distinct Kinase-Independent Role of RIPK3 in CD11c+ Mononuclear Phagocytes in Cytokine-Induced Tissue Repair.Cell Rep. 2017 Mar 7;18(10):2441-2451. doi: 10.1016/j.celrep.2017.02.015. Cell Rep. 2017. PMID: 28273458 Free PMC article.
-
Epidermal Stem Cells in Hair Follicle Cycling and Skin Regeneration: A View From the Perspective of Inflammation.Front Cell Dev Biol. 2020 Nov 9;8:581697. doi: 10.3389/fcell.2020.581697. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33240882 Free PMC article. Review.
-
Vascular endothelial Cdc42 deficiency delays skin wound-healing processes by increasing IL-1β and TNF-α expression.Am J Transl Res. 2019 Jan 15;11(1):257-268. eCollection 2019. Am J Transl Res. 2019. PMID: 30787984 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

