A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- PMID: 26452208
- PMCID: PMC4599908
- DOI: 10.1371/journal.pgen.1005526
A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
Abstract
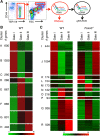
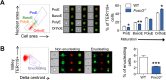
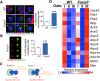
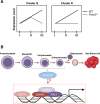
Circulating red blood cells (RBCs) are essential for tissue oxygenation and homeostasis. Defective terminal erythropoiesis contributes to decreased generation of RBCs in many disorders. Specifically, ineffective nuclear expulsion (enucleation) during terminal maturation is an obstacle to therapeutic RBC production in vitro. To obtain mechanistic insights into terminal erythropoiesis we focused on FOXO3, a transcription factor implicated in erythroid disorders. Using an integrated computational and experimental systems biology approach, we show that FOXO3 is essential for the correct temporal gene expression during terminal erythropoiesis. We demonstrate that the FOXO3-dependent genetic network has critical physiological functions at key steps of terminal erythropoiesis including enucleation and mitochondrial clearance processes. FOXO3 loss deregulated transcription of genes implicated in cell polarity, nucleosome assembly and DNA packaging-related processes and compromised erythroid enucleation. Using high-resolution confocal microscopy and imaging flow cytometry we show that cell polarization is impaired leading to multilobulated Foxo3-/- erythroblasts defective in nuclear expulsion. Ectopic FOXO3 expression rescued Foxo3-/- erythroblast enucleation-related gene transcription, enucleation defects and terminal maturation. Remarkably, FOXO3 ectopic expression increased wild type erythroblast maturation and enucleation suggesting that enhancing FOXO3 activity may improve RBCs production. Altogether these studies uncover FOXO3 as a novel regulator of erythroblast enucleation and terminal maturation suggesting FOXO3 modulation might be therapeutic in disorders with defective erythroid maturation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Following Transcriptome to Uncover FOXO Biological Functions.Methods Mol Biol. 2019;1890:219-227. doi: 10.1007/978-1-4939-8900-3_18. Methods Mol Biol. 2019. PMID: 30414157 Free PMC article.
-
Mitochondrial regulation of erythropoiesis in homeostasis and disease.Br J Haematol. 2024 Aug;205(2):429-439. doi: 10.1111/bjh.19600. Epub 2024 Jun 30. Br J Haematol. 2024. PMID: 38946206 Free PMC article. Review.
-
FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis.Am J Hematol. 2014 Oct;89(10):954-63. doi: 10.1002/ajh.23786. Epub 2014 Jul 22. Am J Hematol. 2014. PMID: 24966026 Free PMC article.
-
Investigation of FoxO3 dynamics during erythroblast development in β-thalassemia major.PLoS One. 2017 Nov 3;12(11):e0187610. doi: 10.1371/journal.pone.0187610. eCollection 2017. PLoS One. 2017. PMID: 29099866 Free PMC article.
-
An Overview of Different Strategies to Recreate the Physiological Environment in Experimental Erythropoiesis.Int J Mol Sci. 2020 Jul 24;21(15):5263. doi: 10.3390/ijms21155263. Int J Mol Sci. 2020. PMID: 32722249 Free PMC article. Review.
Cited by
-
Following Transcriptome to Uncover FOXO Biological Functions.Methods Mol Biol. 2019;1890:219-227. doi: 10.1007/978-1-4939-8900-3_18. Methods Mol Biol. 2019. PMID: 30414157 Free PMC article.
-
Iron metabolism under conditions of ineffective erythropoiesis in β-thalassemia.Blood. 2019 Jan 3;133(1):51-58. doi: 10.1182/blood-2018-07-815928. Epub 2018 Nov 6. Blood. 2019. PMID: 30401707 Free PMC article. Review.
-
Transcription factors FOXO in the regulation of homeostatic hematopoiesis.Curr Opin Hematol. 2018 Jul;25(4):290-298. doi: 10.1097/MOH.0000000000000441. Curr Opin Hematol. 2018. PMID: 29782339 Free PMC article. Review.
-
Biological Cells as Therapeutic Delivery Vehicles.Trends Pharmacol Sci. 2021 Feb;42(2):106-118. doi: 10.1016/j.tips.2020.11.008. Epub 2020 Dec 17. Trends Pharmacol Sci. 2021. PMID: 33342562 Free PMC article. Review.
-
Mitochondrial regulation of erythropoiesis in homeostasis and disease.Br J Haematol. 2024 Aug;205(2):429-439. doi: 10.1111/bjh.19600. Epub 2024 Jun 30. Br J Haematol. 2024. PMID: 38946206 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

