Insights into the recent emergence and expansion of eastern equine encephalitis virus in a new focus in the Northern New England USA
- PMID: 26453283
- PMCID: PMC4600208
- DOI: 10.1186/s13071-015-1145-2
Insights into the recent emergence and expansion of eastern equine encephalitis virus in a new focus in the Northern New England USA
Abstract
Background: Eastern equine encephalomyelitis virus (EEEV) causes a highly pathogenic zoonosis that circulates in an enzootic cycle involving the ornithophagic mosquito, Culiseta melanura, and wild passerine birds in freshwater hardwood swamps in the northeastern U.S. Epidemic/epizootic transmission to humans/equines typically occurs towards the end of the transmission season and is generally assumed to be mediated by locally abundant and contiguous mammalophagic "bridge vector" mosquitoes.
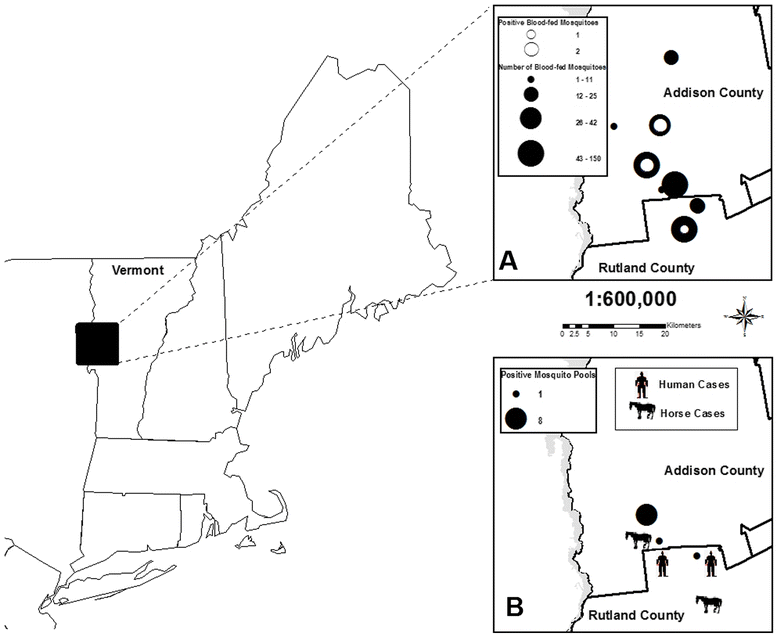
Methods: Engorged mosquitoes were collected using CDC light, resting box, and gravid traps during epidemic transmission of EEEV in 2012 in Addison and Rutland counties, Vermont. Mosquitoes were identified to species and blood meal analysis performed by sequencing mitochondrial cytochrome b gene polymerase chain reaction products. Infection status with EEEV in mosquitoes was determined using cell culture and RT-PCR assays, and all viral isolates were sequenced and compared to other EEEV strains by phylogenetic analysis.
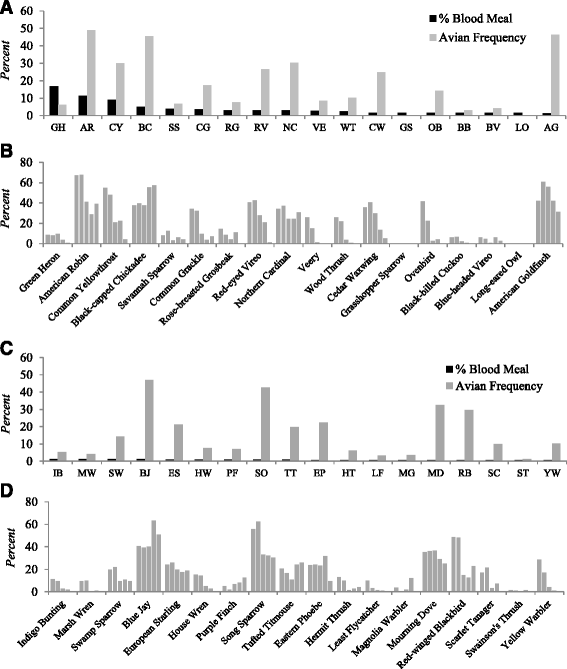
Results: The host choices of 574 engorged mosquitoes were as follows: Cs. melanura (n = 331, 94.3 % avian-derived, 5.7 % mammalian-derived); Anopheles quadrimaculatus (n = 164, 3.0 % avian, 97.0 % mammalian); An. punctipennis (n = 56, 7.2 % avian, 92.8 % mammalian), Aedes vexans (n = 9, 22.2 % avian, 77.8 % mammalian); Culex pipiens s.l. n = 6, 100 % avian); Coquillettidia perturbans (n = 4, 25.0 % avian, 75.0 % mammalian); and Cs. morsitans (n = 4, 100 % avian). A seasonal shift in blood feeding by Cs. melanura from Green Heron towards other avian species was observed. EEEV was successfully isolated from blood-fed Cs. melanura and analyzed by phylogenetic analysis. Vermont strains from 2012 clustered with viral strains previously isolated in Virginia yet were genetically distinct from an earlier EEEV isolate from Vermont during 2011.
Conclusions: Culiseta melanura acquired blood meals primarily from birds and focused feeding activity on several competent species capable of supporting EEEV transmission. Culiseta melanura also occasionally obtained blood meals from mammalian hosts including humans. This mosquito species serves as the primary vector of EEEV among wild bird species, but also is capable of occasionally contributing to epidemic/epizootic transmission of EEEV to humans/equines. Other mosquito species including Cq. perturbans that feed more opportunistically on both avian and mammalian hosts may be important in epidemic/epizootic transmission under certain conditions. Phylogenetic analyses suggest that EEEV was independently introduced into Vermont on at least two separate occasions.
Figures


References
-
- Morris CD. Eastern equine encephalomyelitis. In: Monath TP, editor. The Arboviruses: epidemiology and ecology. Boca Raton, FL: CRC Press; 1988. pp. 1–20.
-
- Hayes RO. Eastern and western encephalitis. In: Beran GW, editor. Viral Zoonoses, Section B. Boca Raton, FL: CRC Handbook Series in Zoonoses; 1981. pp. 29–57.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

