Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex
- PMID: 26453330
- PMCID: PMC4698379
- DOI: 10.1152/ajpheart.00463.2015
Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex
Abstract
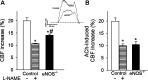
Impairment of moment-to-moment adjustment of cerebral blood flow (CBF) via neurovascular coupling is thought to play a critical role in the genesis of cognitive impairment associated with aging and pathological conditions associated with accelerated cerebromicrovascular aging (e.g., hypertension, obesity). Although previous studies demonstrate that endothelial dysfunction plays a critical role in neurovascular uncoupling in these conditions, the role of endothelial NO mediation in neurovascular coupling responses is not well understood. To establish the link between endothelial function and functional hyperemia, neurovascular coupling responses were studied in mutant mice overexpressing or deficient in endothelial NO synthase (eNOS), and the role of P2Y1 receptors in purinergic glioendothelial coupling was assessed. We found that genetic depletion of eNOS (eNOS(-/-)) and pharmacological inhibition of NO synthesis significantly decreased the CBF responses in the somatosensory cortex evoked by whisker stimulation and by administration of ATP. Overexpression of eNOS enhanced NO mediation of functional hyperemia. In control mice, the selective and potent P2Y1 receptor antagonist MRS2179 attenuated both whisker stimulation-induced and ATP-mediated CBF responses, whereas, in eNOS(-/-) mice, the inhibitory effects of MRS2179 were blunted. Collectively, our findings provide additional evidence for purinergic glio-endothelial coupling during neuronal activity, highlighting the role of ATP-mediated activation of eNOS via P2Y1 receptors in functional hyperemia.
Keywords: astrocyte; dementia; endothelial dysfunction; endothelial nitric oxide synthase; endothelium; vascular cognitive impairment.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Aldasoro M, Martinez C, Vila JM, Medina P, Lluch S. Influence of endothelial nitric oxide on adrenergic contractile responses of human cerebral arteries. J Cereb Blood Flow Metab 16: 623–628, 1996. - PubMed
-
- Ayata C, Ma J, Meng W, Huang P, Moskowitz MA. l-NA-sensitive rCBF augmentation during vibrissal stimulation in type III nitric oxide synthase mutant mice. J Cereb Blood Flow Metab 16: 539–541, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

