Role of IGF-I signaling in muscle bone interactions
- PMID: 26453498
- PMCID: PMC4600536
- DOI: 10.1016/j.bone.2015.04.036
Role of IGF-I signaling in muscle bone interactions
Abstract
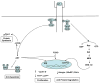
Skeletal muscle and bone rely on a number of growth factors to undergo development, modulate growth, and maintain physiological strength. A major player in these actions is insulin-like growth factor I (IGF-I). However, because this growth factor can directly enhance muscle mass and bone density, it alters the state of the musculoskeletal system indirectly through mechanical crosstalk between these two organ systems. Thus, there are clearly synergistic actions of IGF-I that extend beyond the direct activity through its receptor. This review will cover the production and signaling of IGF-I as it pertains to muscle and bone, the chemical and mechanical influences that arise from IGF-I activity, and the potential for therapeutic strategies based on IGF-I. This article is part of a Special Issue entitled "Muscle Bone Interactions".
Keywords: Bone density; Insulin-like growth factor; Muscle hypertrophy; Regeneration; Repair.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy.J Musculoskelet Neuronal Interact. 2007 Jul-Sep;7(3):208-18. J Musculoskelet Neuronal Interact. 2007. PMID: 17947802 Review.
-
Insulin Resistance Negatively Influences the Muscle-Dependent IGF-1-Bone Mass Relationship in Premenarcheal Girls.J Clin Endocrinol Metab. 2016 Jan;101(1):199-205. doi: 10.1210/jc.2015-3451. Epub 2015 Nov 17. J Clin Endocrinol Metab. 2016. PMID: 26574958 Free PMC article. Clinical Trial.
-
Insulin-Like Growth Factor I Regulation and Its Actions in Skeletal Muscle.Compr Physiol. 2018 Dec 13;9(1):413-438. doi: 10.1002/cphy.c180010. Compr Physiol. 2018. PMID: 30549022 Review.
-
The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair.Appl Physiol Nutr Metab. 2006 Dec;31(6):791-7. doi: 10.1139/h06-054. Appl Physiol Nutr Metab. 2006. PMID: 17213901 Review.
-
The therapeutic potential of IGF-I in skeletal muscle repair.Trends Endocrinol Metab. 2013 Jun;24(6):310-9. doi: 10.1016/j.tem.2013.03.004. Epub 2013 Apr 27. Trends Endocrinol Metab. 2013. PMID: 23628587 Free PMC article. Review.
Cited by
-
Non-alcoholic fatty liver disease connections with fat-free tissues: A focus on bone and skeletal muscle.World J Gastroenterol. 2017 Mar 14;23(10):1747-1757. doi: 10.3748/wjg.v23.i10.1747. World J Gastroenterol. 2017. PMID: 28348479 Free PMC article. Review.
-
Betaine promotes cell differentiation of human osteoblasts in primary culture.J Transl Med. 2017 Jun 7;15(1):132. doi: 10.1186/s12967-017-1233-5. J Transl Med. 2017. PMID: 28592272 Free PMC article.
-
Effect of β-casein A2 cow milk supplementation on physical growth, inflammation, growth-related hormones, and nutritional biomarkers in stunted children.Ann Pediatr Endocrinol Metab. 2025 Jun;30(3):119-126. doi: 10.6065/apem.2448158.079. Epub 2025 Feb 25. Ann Pediatr Endocrinol Metab. 2025. PMID: 39999830 Free PMC article.
-
Associations between serum levels of insulin-like growth factor-I and bone mineral acquisition in pubertal children: a 3-year follow-up study in Hamamatsu, Japan.J Physiol Anthropol. 2019 Dec 5;38(1):16. doi: 10.1186/s40101-019-0210-5. J Physiol Anthropol. 2019. PMID: 31806017 Free PMC article.
-
Bone-organ axes: bidirectional crosstalk.Mil Med Res. 2024 Jun 12;11(1):37. doi: 10.1186/s40779-024-00540-9. Mil Med Res. 2024. PMID: 38867330 Free PMC article. Review.
References
-
- Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. The Journal of biological chemistry. 1986;261:4828–4832. - PubMed
-
- Shimatsu A, Rotwein P. Mosaic evolution of the insulin-like growth factors. Organization, sequence, and expression of the rat insulin-like growth factor I gene. J Biol Chem. 1987;262:7894–7900. - PubMed
-
- Adamo ML, Neuenschwander S, LeRoith D, Roberts CT. Structure, Expression, And Regulation Of The IGF-1 Gene. Current Directions in Insulin-Like Growth Factor Research. 1994:1–11. Capter.
-
- Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2006;31:791–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

