Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study
- PMID: 26453639
- PMCID: PMC4893084
- DOI: 10.1136/bjophthalmol-2015-307249
Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study
Abstract
Aims: To demonstrate non-inferiority of ranibizumab treat-and-extend (T&E) with/without laser to ranibizumab pro re nata (PRN) for best-corrected visual acuity (BCVA) in patients with diabetic macular oedema (DMO).
Methods: A 24-month single-masked study with patients randomised 1:1:1 to T&E+laser (n=121), T&E (n=128) or PRN (control; n=123). All patients received monthly injections until BCVA stabilisation. The investigator decided on re-treatment in the PRN and treatment-interval adaptations in the T&E groups based on loss of BCVA stability due to DMO activity. Likewise, laser treatment was at investigator's discretion. Collectively, these features reflect a real-life scenario. Endpoints included mean average change in BCVA from baseline to months 1-12 (primary), mean BCVA change from baseline to months 12 and 24, treatment exposure and safety profile.
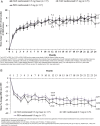
Results: Both T&E regimens were non-inferior to PRN based on mean average BCVA change from baseline to months 1-12 (T&E+laser: +5.9 and T&E: +6.1 vs PRN: +6.2 letters; both p<0.0001). Mean BCVA change at month 24 was similar across groups (+8.3, +6.5 and +8.1 letters, respectively). The mean number of injections was 12.4 and 12.8 in the T&E+laser and T&E groups and 10.7 in the PRN group. The T&E regimens showed 46% reduction in the number of clinic visits. Over 70% of patients maintained their BCVA, with treatment intervals of ≥2 months over 24 months. Safety profile was consistent with that described in the product information.
Conclusions: T&E is a feasible treatment option for patients with DMO, with a potential to reduce treatment burden. Slightly more injections were required versus PRN, likely due to the specifics of the T&E regimen applied here.
Trial registration number: NCT01171976.
Keywords: Clinical Trial; Macula; Treatment Lasers; Treatment Medical; Vision.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
-
- Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology 1984;91:1–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
