Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa
- PMID: 26453676
- PMCID: PMC4643530
- DOI: 10.1093/humrep/dev243
Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa
Abstract
Study question: Are significant abnormalities of CatSper function present in IVF patients with normal sperm concentration and motility and if so what is their functional significance for fertilization success?
Summary answer: Sperm with a near absence of CatSper current failed to respond to activation of CatSper by progesterone and there was fertilization failure at IVF.
What is known already: In human spermatozoa, Ca(2+) influx induced by progesterone is mediated by CatSper, a sperm-specific Ca(2+) channel. A suboptimal Ca(2+) influx is significantly associated with, and more prevalent in, men with abnormal semen parameters, and is associated with reduced fertilizing capacity. However, abnormalities in CatSper current can only be assessed directly using electrophysiology. There is only one report of a CatSper-deficient man who showed no progesterone potentiated CatSper current. A CatSper 2 genetic abnormality was present but there was no information on the [Ca(2+)]i response to CatSper activation by progesterone. Additionally, the semen samples had indicating significant abnormalities (oligoasthenoteratozoospermia) multiple suboptimal functional responses in the spermatozoon. As such it cannot be concluded that impaired CatSper function alone causes infertility or that CatSper blockade is a potential safe target for contraception.
Study design, size, duration: Spermatozoa were obtained from donors and subfertile IVF patients attending a hospital assisted reproductive techniques clinic between January 2013 and December 2014. In total 134 IVF patients, 28 normozoospermic donors and 10 patients recalled due to a history of failed/low fertilization at IVF took part in the study.
Participants/materials, setting, methods: Samples were primarily screened using the Ca(2+) influx induced by progesterone and, if cell number was sufficient, samples were also assessed by hyperactivation and penetration into viscous media. A defective Ca(2+) response to progesterone was defined using the 99% confidence interval from the distribution of response amplitudes in normozoospermic donors. Samples showing a defective Ca(2+) response were further examined in order to characterize the potential CatSper abnormalities. In men where there was a consistent and robust failure of calcium signalling, a direct assessment of CatSper function was performed using electrophysiology (patch clamping), and a blood sample was obtained for genetic analysis.
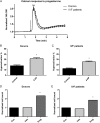
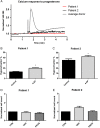
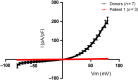
Main results and the role of chance: A total of 101/102 (99%) IVF patients and 22/23 (96%) donors exhibited a normal Ca(2+) response. The mean (± SD) normalized peak response did not differ between donors and IVF patients (2.57 ± 0.68 [n = 34 ejaculates from 23 different donors] versus 2.66 ± 0.68 [n = 102 IVF patients], P = 0.63). In recall patients, 9/10 (90%) showed a normal Ca(2+) response. Three men were initially identified with a defective Ca(2+) influx. However, only one (Patient 1) had a defective response in repeat semen samples. Electrophysiology experiments on sperm from Patient 1 showed a near absence of CatSper current and exon screening demonstrated no mutations in the coding regions of the CatSper complex. There was no increase in penetration of viscous media when the spermatozoa were stimulated with progesterone and importantly there was failed fertilization at IVF.
Limitations, reasons for caution: A key limitation relates to working with a specific functional parameter (Ca(2+) influx induced by progesterone) in fresh sperm samples from donors and patients that have limited viability. Therefore, for practical, technical and logistical reasons, some men (∼ 22% of IVF patients) could not be screened. As such the incidence of significant Ca(2+) abnormalities induced by progesterone may be higher than the ∼ 1% observed here. Additionally, we used a strict definition of a defective Ca(2+) influx such that only substantial abnormalities were selected for further study. Furthermore, electrophysiology was only performed on one patient with a robust and repeatable defective calcium response. This man had negligible CatSper current but more subtle abnormalities (e.g. currents present but significantly smaller) may have been present in men with either normal or below normal Ca(2+) influx.
Wider implications of the findings: These data add significantly to the understanding of the role of CatSper in human sperm function and its impact on male fertility. Remarkably, these findings provide the first direct evidence that CatSper is a suitable and specific target for human male contraception.
Keywords: CatSper; calcium stores; contraception; electrophysiology; failed fertilization; ion channels; male fertility; sperm dysfunction; sperm motility; unexplained infertility.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures
Similar articles
-
Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF.Hum Reprod. 2016 Jun;31(6):1147-57. doi: 10.1093/humrep/dew056. Epub 2016 Apr 6. Hum Reprod. 2016. PMID: 27052499 Free PMC article.
-
The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation.Hum Reprod. 2013 Apr;28(4):866-76. doi: 10.1093/humrep/des467. Epub 2013 Feb 12. Hum Reprod. 2013. PMID: 23406974 Free PMC article.
-
Single-cell analysis of [Ca2+]i signalling in sub-fertile men: characteristics and relation to fertilization outcome.Hum Reprod. 2018 Jun 1;33(6):1023-1033. doi: 10.1093/humrep/dey096. Hum Reprod. 2018. PMID: 29697805 Free PMC article.
-
Progesterone, spermatozoa and reproduction: An updated review.Mol Cell Endocrinol. 2020 Oct 1;516:110952. doi: 10.1016/j.mce.2020.110952. Epub 2020 Jul 24. Mol Cell Endocrinol. 2020. PMID: 32712385 Review.
-
CatSper: The complex main gate of calcium entry in mammalian spermatozoa.Mol Cell Endocrinol. 2020 Dec 1;518:110951. doi: 10.1016/j.mce.2020.110951. Epub 2020 Jul 24. Mol Cell Endocrinol. 2020. PMID: 32712386 Review.
Cited by
-
The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation?Int J Mol Sci. 2023 Sep 6;24(18):13750. doi: 10.3390/ijms241813750. Int J Mol Sci. 2023. PMID: 37762052 Free PMC article. Review.
-
UV filters in matched seminal fluid-, urine-, and serum samples from young men.J Expo Sci Environ Epidemiol. 2021 Mar;31(2):345-355. doi: 10.1038/s41370-020-0209-3. Epub 2020 Feb 12. J Expo Sci Environ Epidemiol. 2021. PMID: 32051500
-
Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF.Hum Reprod. 2016 Jun;31(6):1147-57. doi: 10.1093/humrep/dew056. Epub 2016 Apr 6. Hum Reprod. 2016. PMID: 27052499 Free PMC article.
-
The Detection of CatSper1 and CatSper3 Expression in Men with Normozoospermia and Asthenoteratozoospermia and Its Association with Sperm Parameters, Fertilization Rate, Embryo Quality.Reprod Sci. 2024 Mar;31(3):704-713. doi: 10.1007/s43032-023-01397-4. Epub 2023 Nov 13. Reprod Sci. 2024. PMID: 37957468
-
The Role of Sperm Membrane Potential and Ion Channels in Regulating Sperm Function.Int J Mol Sci. 2023 Apr 10;24(8):6995. doi: 10.3390/ijms24086995. Int J Mol Sci. 2023. PMID: 37108159 Free PMC article. Review.
References
-
- Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod 2013;19:785–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

