Phenotypic and Morphological Properties of Germinal Center Dark Zone Cxcl12-Expressing Reticular Cells
- PMID: 26453751
- PMCID: PMC4637241
- DOI: 10.4049/jimmunol.1501191
Phenotypic and Morphological Properties of Germinal Center Dark Zone Cxcl12-Expressing Reticular Cells
Abstract
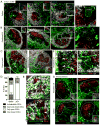
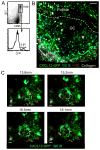
The germinal center (GC) is divided into a dark zone (DZ) and a light zone (LZ). GC B cells must cycle between these zones to achieve efficient Ab affinity maturation. Follicular dendritic cells (FDCs) are well characterized for their role in supporting B cell Ag encounter in primary follicles and in the GC LZ. However, the properties of stromal cells supporting B cells in the DZ are relatively unexplored. Recent work identified a novel stromal population of Cxcl12-expressing reticular cells (CRCs) in murine GC DZs. In this article, we report that CRCs have diverse morphologies, appearing in open and closed networks, with variable distribution in lymphoid tissue GCs. CRCs are also present in splenic and peripheral lymph node primary follicles. Real-time two-photon microscopy of Peyer's patch GCs demonstrates B cells moving in close association with CRC processes. CRCs are gp38(+) with low to undetectable expression of FDC markers, but CRC-like cells in the DZ are lineage marked, along with FDCs and fibroblastic reticular cells, by CD21-Cre- and Ccl19-Cre-directed fluorescent reporters. In contrast to FDCs, CRCs do not demonstrate dependence on lymphotoxin or TNF for chemokine expression or network morphology. CRC distribution in the DZ does require CXCR4 signaling, which is necessary for GC B cells to access the DZ and likely to interact with CRC processes. Our findings establish CRCs as a major stromal cell type in the GC DZ and suggest that CRCs support critical activities of GC B cells in the DZ niche through Cxcl12 expression and direct cell-cell interactions.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures




References
-
- Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunological Reviews. 2000;176:181–193. - PubMed
-
- Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

