Senescence and cancer: An evolving inflammatory paradox
- PMID: 26453912
- PMCID: PMC4733607
- DOI: 10.1016/j.bbcan.2015.10.001
Senescence and cancer: An evolving inflammatory paradox
Abstract
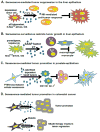
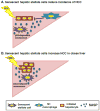
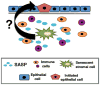
The senescent phenotype was first described in 1961 as a phenomenon characterized by the cessation of cellular division. After years of debate as to whether it represented a tissue culture artifact or an important biological process, it is now appreciated that senescence plays an important role in tumorigenesis. Further, senescence is integral to normal biological processes such as embryogenesis and the maintenance of tissue homeostasis. Now with defined roles in development, wound healing, tumor promotion and tumor suppression, it is not surprising that attention has turned to refining our understanding of the mechanisms behind, and consequences of, the induction of senescence. One emerging role for senescence lies in the ability of senescence to orchestrate an inflammatory response: factors secreted by senescent cells have been identified in multiple contexts to modulate various aspects of the immune response. As with many of the previously described roles for senescence, the type of inflammation established by the senescence phenotype is varied and dependent on context. In this review, we discuss the current state of the field with a focus on the paradoxical outcomes of the senescence-induced inflammatory responses in the context of cancer. A more complete understanding of senescence and an appreciation for its complexities will be important for eventual development of senescence-targeted therapies.
Keywords: Cancer; Immune cell; Inflammation; Senescence.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
References
-
- Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes & development. 1994;8:2540–51. - PubMed
-
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. - PubMed
-
- Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer research. 2006;66:2881–4. - PubMed
-
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–31. - PubMed
-
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

