DDX3 functions in antiviral innate immunity through translational control of PACT
- PMID: 26454002
- PMCID: PMC7164078
- DOI: 10.1111/febs.13553
DDX3 functions in antiviral innate immunity through translational control of PACT
Abstract
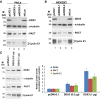
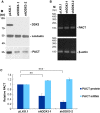
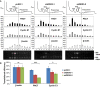
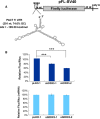
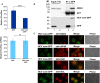
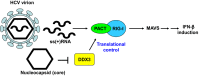
It has emerged that DDX3 plays a role in antiviral innate immunity. However, the exact mechanism by which DDX3 functions in antiviral innate immunity remains to be determined. We found that the expression of the protein activator of the interferon-induced protein kinase (PACT) was regulated by DDX3 in human cells. PACT acts as a cellular activator of retinoic acid-inducible gene-I-like receptors in the sensing of viral RNAs. DDX3 facilitated the translation of PACT mRNA that may contain a structured 5' UTR. Knockdown of DDX3 decreased the viral RNA detection sensitivity of the cells. PACT partially rescued defects of interferon-β1 and chemokine (C-C motif) ligand 5/RANTES (regulated on activation normal T cell expressed and secreted) induction in DDX3-knockdown HEK293 cells. Therefore, DDX3 may participate in antiviral innate immunity, at least in part, by translational control of PACT. Moreover, we show that overexpression of the hepatitis C virus (HCV) core protein inhibited the translation of a reporter mRNA harboring the PACT 5' UTR. The HCV core protein was associated and colocalized with DDX3 in cytoplasmic stress granules, suggesting that the HCV core may abrogate the function of DDX3 by sequestering DDX3 in stress granules. The perturbation of DDX3 by viral proteins delineates a critical role for DDX3 in antiviral host defense.
Keywords: DDX3; PACT; antiviral innate immunity; hepatitis C virus; translational control.
© 2015 FEBS.
Figures

References
-
- Cordin O, Banroques J, Tanner NK & Linder P (2006) The DEAD‐box protein family of RNA helicases. Gene 367, 17–37. - PubMed
-
- Rocak S & Linder P (2004) DEAD‐box proteins: the driving forces behind RNA metabolism. Nat Rev Mol Cell Biol 5, 232–241. - PubMed
-
- Chao CH, Chen CM, Cheng PL, Shih JW, Tsou AP & Lee YH (2006) DDX3, a DEAD box RNA helicase with tumor growth‐suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res 66, 6579–6588. - PubMed
-
- Jamieson DJ, Rahe B, Pringle J & Beggs JD (1991) A suppressor of a yeast splicing mutation (prp8‐1) encodes a putative ATP‐dependent RNA helicase. Nature 349, 715–717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

