Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: Ion transport effects and metabolism of butter flavoring agents
- PMID: 26454031
- PMCID: PMC4739639
- DOI: 10.1016/j.taap.2015.10.004
Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: Ion transport effects and metabolism of butter flavoring agents
Abstract
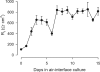
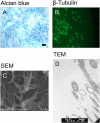
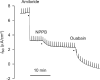
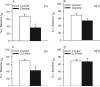
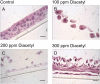
Inhalation of butter flavoring by workers in the microwave popcorn industry may result in “popcorn workers' lung.” In previous in vivo studies rats exposed for 6 h to vapor from the flavoring agents, diacetyl and 2,3-pentanedione, acquired flavoring concentration-dependent damage of the upper airway epithelium and airway hyporeactivity to inhaled methacholine. Because ion transport is essential for lung fluid balance,we hypothesized that alterations in ion transport may be an early manifestation of butter flavoring-induced toxicity.We developed a system to expose cultured human bronchial/tracheal epithelial cells (NHBEs) to flavoring vapors. NHBEs were exposed for 6 h to diacetyl or 2,3-pentanedione vapors (25 or ≥ 60 ppm) and the effects on short circuit current and transepithelial resistance (Rt) were measured. Immediately after exposure to 25 ppm both flavorings reduced Na+ transport,without affecting Cl- transport or Na+,K+-pump activity. Rt was unaffected. Na+ transport recovered 18 h after exposure. Concentrations (100-360 ppm) of diacetyl and 2,3-pentanedione reported earlier to give rise in vivo to epithelial damage, and 60 ppm, caused death of NHBEs 0 h post-exposure. Analysis of the basolateral medium indicated that NHBEs metabolize diacetyl and 2,3-pentanedione to acetoin and 2-hydroxy-3-pentanone, respectively. The results indicate that ion transport is inhibited transiently in airway epithelial cells by lower concentrations of the flavorings than those that result in morphological changes of the cells in vivo or in vitro.
Keywords: 2,3-Pentanedione; Diacetyl; Dicarbonyl/l-xylulose reductase; Human airway epithelial cells; Ion transport; Vapor exposure.
Figures





References
-
- Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur. Respir. J. 2004;24:298–302. - PubMed
-
- Borders CL, Jr., Riordan JF. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975;14:4699–4704. - PubMed
-
- Calam E, Porté S, Fernández MR, Farrés J, Parés X, Biosca JA. Biocatalytic production of alpha-hydroxy ketones and vicinal diols by yeast and human aldo-keto reductases. Chem. Biol. Interact. 2013;202:195–203. - PubMed
-
- Cichocki JA, Smith GJ, Morris JB. Tissue sensitivity of the rat upper and lower extrapulmonary airways to the inhaled electrophilic air pollutants diacetyl and acrolein. Toxicol. Sci. 2014;142:126–136. - PubMed
-
- Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am. J. Physiol. Lung-C. 2002;282:L226–L236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

