Redox-Active Mn Porphyrin-based Potent SOD Mimic, MnTnBuOE-2-PyP(5+), Enhances Carbenoxolone-Mediated TRAIL-Induced Apoptosis in Glioblastoma Multiforme
- PMID: 26454429
- PMCID: PMC4926165
- DOI: 10.1007/s12015-015-9628-2
Redox-Active Mn Porphyrin-based Potent SOD Mimic, MnTnBuOE-2-PyP(5+), Enhances Carbenoxolone-Mediated TRAIL-Induced Apoptosis in Glioblastoma Multiforme
Abstract
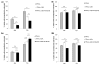
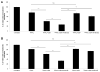
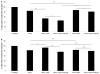
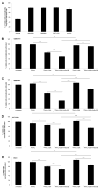
Glioblastoma multiforme is the most malignant tumor of the brain and is challenging to treat due to its highly invasive nature and heterogeneity. Malignant brain tumor displays high metabolic activity which perturbs its redox environment and in turn translates to high oxidative stress. Thus, pushing the oxidative stress level to achieve the maximum tolerable threshold that induces cell death is a potential strategy for cancer therapy. Previously, we have shown that gap junction inhibitor, carbenoxolone (CBX), is capable of enhancing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) -induced apoptosis in glioma cells. Since CBX is known to induce oxidative stress, we hypothesized that the addition of another potent mediator of oxidative stress, powerful SOD mimic MnTnBuOE-2-PyP(5+) (MnBuOE), could further enhance TRAIL-driven therapeutic efficacy in glioma cells. Our results showed that combining TRAIL + CBX with MnBuOE significantly enhances cell death of glioma cell lines and this enhancement could be further potentiated by CBX pretreatment. MnBuOE-driven cytotoxicity is due to its ability to take advantage of oxidative stress imposed by CBX + TRAIL system, and enhance it in the presence of endogenous reductants, ascorbate and thiol, thereby producing cytotoxic H2O2, and in turn inducing death of glioma cells but not normal astrocytes. Most importantly, combination treatment significantly reduces viability of TRAIL-resistant Asian patient-derived glioma cells, thus demonstrating the potential clinical use of our therapeutic system. It was reported that H2O2 is involved in membrane depolarization-based sensitization of cancer cells toward TRAIL. MnBuOE is entering Clinical Trials as a normal brain radioprotector in glioma patients at Duke University increasing Clinical relevance of our studies.
Keywords: Ascorbate; Carbenoxolone; Glioma; Manganese porphyrin; NAC; SOD mimic; TRAIL-modified human mesenchymal stem cells.
Conflict of interest statement
Figures


Similar articles
-
Anticancer therapeutic potential of Mn porphyrin/ascorbate system.Free Radic Biol Med. 2015 Dec;89:1231-47. doi: 10.1016/j.freeradbiomed.2015.10.416. Epub 2015 Oct 20. Free Radic Biol Med. 2015. PMID: 26496207 Free PMC article.
-
Carbenoxolone enhances TRAIL-induced apoptosis through the upregulation of death receptor 5 and inhibition of gap junction intercellular communication in human glioma.Stem Cells Dev. 2013 Jul 1;22(13):1870-82. doi: 10.1089/scd.2012.0529. Epub 2013 Mar 26. Stem Cells Dev. 2013. PMID: 23428290 Free PMC article.
-
Celastrol enhances TRAIL-induced apoptosis in human glioblastoma via the death receptor pathway.Cancer Chemother Pharmacol. 2019 Oct;84(4):719-728. doi: 10.1007/s00280-019-03900-8. Epub 2019 Jul 8. Cancer Chemother Pharmacol. 2019. PMID: 31281953
-
Overcoming TRAIL Resistance for Glioblastoma Treatment.Biomolecules. 2021 Apr 14;11(4):572. doi: 10.3390/biom11040572. Biomolecules. 2021. PMID: 33919846 Free PMC article. Review.
-
A combination of two antioxidants (an SOD mimic and ascorbate) produces a pro-oxidative effect forcing Escherichia coli to adapt via induction of oxyR regulon.Anticancer Agents Med Chem. 2011 May 1;11(4):329-40. doi: 10.2174/187152011795677562. Anticancer Agents Med Chem. 2011. PMID: 21355843 Free PMC article. Review.
Cited by
-
Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity.Antioxidants (Basel). 2022 Nov 30;11(12):2375. doi: 10.3390/antiox11122375. Antioxidants (Basel). 2022. PMID: 36552581 Free PMC article. Review.
-
Anticancer therapeutic potential of Mn porphyrin/ascorbate system.Free Radic Biol Med. 2015 Dec;89:1231-47. doi: 10.1016/j.freeradbiomed.2015.10.416. Epub 2015 Oct 20. Free Radic Biol Med. 2015. PMID: 26496207 Free PMC article.
-
Balanced Duality: H2O2-Based Therapy in Cancer and Its Protective Effects on Non-Malignant Tissues.Int J Mol Sci. 2024 Aug 15;25(16):8885. doi: 10.3390/ijms25168885. Int J Mol Sci. 2024. PMID: 39201571 Free PMC article. Review.
-
Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer.Antioxid Redox Signal. 2018 Nov 1;29(13):1237-1272. doi: 10.1089/ars.2017.7485. Epub 2018 Feb 21. Antioxid Redox Signal. 2018. PMID: 29325444 Free PMC article. Review.
-
The Addition of Manganese Porphyrins during Radiation Inhibits Prostate Cancer Growth and Simultaneously Protects Normal Prostate Tissue from Radiation Damage.Antioxidants (Basel). 2018 Jan 25;7(1):21. doi: 10.3390/antiox7010021. Antioxidants (Basel). 2018. PMID: 29370088 Free PMC article.
References
-
- Ho IA, Lam PY. Signaling molecules and pathways involved in MSC tumor tropism. Histology and Histopathology. 2013;28:1427–1438. - PubMed
-
- Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Research. 2005;65:3307–3318. - PubMed
-
- Kang SG, Jeun SS, Lim JY, Kim SM, Yang YS, Oh WI, Huh PW, Park CK. Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Child’s Nervous System. 2008;24:293–302. - PubMed
-
- Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park SH, Sung YC, Jeun SS. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Research. 2008;68:9614–9623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

