The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing
- PMID: 26454445
- PMCID: PMC5002945
- DOI: 10.1007/s00428-015-1858-9
The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing
Abstract
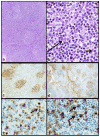
Plasmacytic differentiation may occur in almost all small B cell lymphomas (SBLs), although it varies from being uniformly present (as in lymphoplasmacytic lymphoma (LPL)) to very uncommon (as in mantle cell lymphomas (MCLs)). The discovery of MYD88 L265P mutations in the vast majority of LPLs has had a major impact on the study of these lymphomas. Review of the cases contributed to the 2014 European Association for Haematopathology/Society for Hematopathology slide workshop illustrated how mutational testing has helped refine the diagnostic criteria for LPL, emphasizing the importance of identifying a clonal monotonous lymphoplasmacytic population and highlighting how LPL can still be diagnosed with extensive nodal architectural effacement, very subtle plasmacytic differentiation, follicular colonization, or uncommon phenotypes such as CD5 or CD10 expression. MYD88 L265P mutations were found in 11/11 LPL cases versus only 2 of 28 other SBLs included in its differential diagnosis. Mutational testing also helped to exclude other cases that would have been considered LPL in the past. The workshop also highlighted how plasmacytic differentiation can occur in chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, SOX11 negative MCL, and particularly in marginal zone lymphomas, all of which can cause diagnostic confusion with LPL. The cases also highlighted the difficulty in distinguishing lymphomas with marked plasmacytic differentiation from plasma cell neoplasms. Some SBLs with plasmacytic differentiation can be associated with amyloid, other immunoglobulin deposition, or crystal-storing histiocytosis, which may obscure the underlying neoplasm. Finally, although generally indolent, LPL may transform, with the workshop cases suggesting a role for TP53 abnormalities.
Keywords: Chronic lymphocytic leukemia; Follicular lymphoma; Lymphoplasmacytic lymphoma; MYD88; Mantle cell lymphoma; Marginal zone lymphoma; Plasmacytic differentiation.
Figures










References
-
- Andriko JA, Swerdlow SH, Aguilera NI, Abbondanzo SL. Is lymphoplasmacytic lymphoma/immunocytoma a distinct entity? A clinicopathologic study of 20 cases. Am J Surg Pathol. 2001;25:742–751. - PubMed
-
- Bob R, Falini B, Marafioti T, Paterson JC, Pileri S, Stein H. Nodal reactive and neoplastic proliferation of monocytoid and marginal zone B cells: an immunoarchitectural and molecular study highlighting the relevance of IRTA1 and T-bet as positive markers. Histopathology. 2013;63:482–498. doi: 10.1111/his.12160. - DOI - PubMed
-
- Campo E, Miquel R, Krenacs L, Sorbara L, Raffeld M, Jaffe ES. Primary nodal marginal zone lymphomas of splenic and MALT type. Am J Surg Pathol. 1999;23:59–68. - PubMed
-
- Cheuk W, Yuen HK, Chan AC, Shih LY, Kuo TT, Ma MW, Lo YF, Chan WK, Chan JK. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:1159–1167. doi: 10.1097/PAS.0b013e31816148ad. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

