Memantine improves buprenorphine/naloxone treatment for opioid dependent young adults
- PMID: 26454835
- PMCID: PMC4652072
- DOI: 10.1016/j.drugalcdep.2015.09.020
Memantine improves buprenorphine/naloxone treatment for opioid dependent young adults
Abstract
Background: Opioid use disorders are considered a serious public health problem among young adults. Current treatment is limited to long-term opioid substitution therapy, with high relapse rates after discontinuation. This study evaluated the co-administration of memantine to brief buprenorphine pharmacotherapy as a treatment alternative.
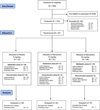
Methods: 13-week double-blind placebo-controlled trial evaluating 80 young adult opioid dependent participants treated with buprenorphine/naloxone 16-4mg/day and randomized to memantine (15mg or 30mg) or placebo. Primary outcomes were a change in the weekly mean proportion of opioid use, and cumulative abstinence rates after rapid buprenorphine discontinuation on week 9.
Results: Treatment retention was not significantly different between groups. The memantine 30mg group was significantly less likely to relapse and to use opioids after buprenorphine discontinuation. Among participants abstinent on week 8, those in the memantine 30mg group (81.9%) were significantly less likely to relapse after buprenorphine was discontinued compared to the placebo group (30%) (p<0.025). Also, the memantine 30mg group had significantly reduced opioid use (mean=0, SEM±0.00) compared to the placebo group (mean=0.33, SEM±0.35; p<0.004) during the last 2 weeks of study participation.
Conclusions: Memantine 30mg significantly improved short-term treatment with buprenorphine/naloxone for opioid dependent young adults by reducing relapse and opioid use after buprenorphine discontinuation. Combined short-term treatment with buprenorphine/naloxone may be an effective alternative treatment to long-term methadone or buprenorphine maintenance in young adults.
Keywords: Buprenorphine; Early relapse; Memantine; Opioid dependence; Young adults.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures



References
-
- Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst. Rev. 2005:CD003154. - PubMed
-
- Beister A, Kraus P, Kuhn W, Dose M, Weindl A, Gerlach M. The N-methyl-D-aspartate antagonist memantine retards progression of Huntington's disease. J. Neural Transm. Suppl. 2004:117–122. - PubMed
-
- Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology (Berl.) 2001;157:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

