Unbiased View of Synaptic and Neuronal Gene Complement in Ctenophores: Are There Pan-neuronal and Pan-synaptic Genes across Metazoa?
- PMID: 26454853
- PMCID: PMC4836450
- DOI: 10.1093/icb/icv104
Unbiased View of Synaptic and Neuronal Gene Complement in Ctenophores: Are There Pan-neuronal and Pan-synaptic Genes across Metazoa?
Abstract
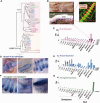
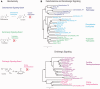
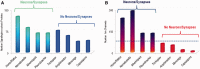
Hypotheses of origins and evolution of neurons and synapses are controversial, mostly due to limited comparative data. Here, we investigated the genome-wide distribution of the bilaterian "synaptic" and "neuronal" protein-coding genes in non-bilaterian basal metazoans (Ctenophora, Porifera, Placozoa, and Cnidaria). First, there are no recognized genes uniquely expressed in neurons across all metazoan lineages. None of the so-called pan-neuronal genes such as embryonic lethal abnormal vision (ELAV), Musashi, or Neuroglobin are expressed exclusively in neurons of the ctenophore Pleurobrachia. Second, our comparative analysis of about 200 genes encoding canonical presynaptic and postsynaptic proteins in bilaterians suggests that there are no true "pan-synaptic" genes or genes uniquely and specifically attributed to all classes of synapses. The majority of these genes encode receptive and secretory complexes in a broad spectrum of eukaryotes. Trichoplax (Placozoa) an organism without neurons and synapses has more orthologs of bilaterian synapse-related/neuron-related genes than do ctenophores-the group with well-developed neuronal and synaptic organization. Third, the majority of genes encoding ion channels and ionotropic receptors are broadly expressed in unicellular eukaryotes and non-neuronal tissues in metazoans. Therefore, they cannot be viewed as neuronal markers. Nevertheless, the co-expression of multiple types of ion channels and receptors does correlate with the presence of neural and synaptic organization. As an illustrative example, the ctenophore genomes encode a greater diversity of ion channels and ionotropic receptors compared with the genomes of the placozoan Trichoplax and the demosponge Amphimedon. Surprisingly, both placozoans and sponges have a similar number of orthologs of "synaptic" proteins as we identified in the genomes of two ctenophores. Ctenophores have a distinct synaptic organization compared with other animals. Our analysis of transcriptomes from 10 different ctenophores did not detect recognized orthologs of synthetic enzymes encoding several classical, low-molecular-weight (neuro)transmitters; glutamate signaling machinery is one of the few exceptions. Novel peptidergic signaling molecules were predicted for ctenophores, together with the diversity of putative receptors including SCNN1/amiloride-sensitive sodium channel-like channels, many of which could be examples of a lineage-specific expansion within this group. In summary, our analysis supports the hypothesis of independent evolution of neurons and, as corollary, a parallel evolution of synapses. We suggest that the formation of synaptic machinery might occur more than once over 600 million years of animal evolution.
© The Author 2015. Published by Oxford University Press on behalf of the Society for Integrative and Comparative Biology. All rights reserved. For permissions please email: journals.permissions@oup.com.
Figures




References
-
- Abascal F, Zardoya R. 2013. Evolutionary analyses of gap junction protein families. Biochim Biophys Acta 1828:4–14. - PubMed
-
- Anctil M. 1985. Cholinergic and monoaminergic mechanisms associated with control of bioluminescence in the ctenophore Mnemiopsis leidyi. J Exp Biol 119:225–38.
-
- Anctil M. 2009. Chemical transmission in the sea anemone Nematostella vectensis: A genomic perspective. Comp Biochem Physiol Part D Genomics Proteomics 4:268–89. - PubMed
-
- Anne C, Gasnier B. 2014. Vesicular neurotransmitter transporters: Mechanistic aspects. Curr Top Membr 73:149–74. - PubMed
-
- Bang ML, Owczarek S. 2013. A matter of balance: Role of neurexin and neuroligin at the synapse. Neurochem Res 38:1174–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

