Wall mechanics and exocytosis define the shape of growth domains in fission yeast
- PMID: 26455310
- PMCID: PMC4618311
- DOI: 10.1038/ncomms9400
Wall mechanics and exocytosis define the shape of growth domains in fission yeast
Abstract
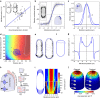
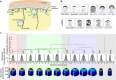
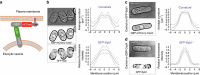
The amazing structural variety of cells is matched only by their functional diversity, and reflects the complex interplay between biochemical and mechanical regulation. How both regulatory layers generate specifically shaped cellular domains is not fully understood. Here, we report how cell growth domains are shaped in fission yeast. Based on quantitative analysis of cell wall expansion and elasticity, we develop a model for how mechanics and cell wall assembly interact and use it to look for factors underpinning growth domain morphogenesis. Surprisingly, we find that neither the global cell shape regulators Cdc42-Scd1-Scd2 nor the major cell wall synthesis regulators Bgs1-Bgs4-Rgf1 are reliable predictors of growth domain geometry. Instead, their geometry can be defined by cell wall mechanics and the cortical localization pattern of the exocytic factors Sec6-Syb1-Exo70. Forceful re-directioning of exocytic vesicle fusion to broader cortical areas induces proportional shape changes to growth domains, demonstrating that both features are causally linked.
Figures



References
-
- Caspar D. L. & Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol. 27, 1–24 (1962). - PubMed
-
- Thompson D. W. On Growth and Form (Cambridge University Press, 1917).
-
- Llorente J. M. G., Hernández-Rojas J. & Bretón J. A minimal representation of the self-assembly of virus capsids. Soft Matter 10, 3560–3569 (2014). - PubMed
-
- Lecuit T. & Lenne P.-F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8, 633–644 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

