Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy
- PMID: 26455329
- PMCID: PMC4618760
- DOI: 10.1039/c5nr04831a
Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy
Abstract
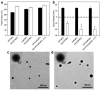
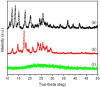
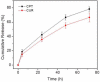
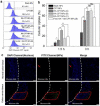
Nanoparticle (NP)-based combination chemotherapy has been proposed as an effective strategy for achieving synergistic effects and targeted drug delivery for colon cancer therapy. Here, we fabricated a series of hyaluronic acid (HA)-functionalized camptothecin (CPT)/curcumin (CUR)-loaded polymeric NPs (HA-CPT/CUR-NPs) with various weight ratios of CPT to CUR (1 : 1, 2 : 1 and 4 : 1). The resultant spherical HA-CPT/CUR-NPs had a desirable particle size (around 289 nm), relative narrow size distribution, and slightly negative zeta potential. These NPs exhibited a simultaneous sustained release profile for both drugs throughout the time frame examined. Subsequent cellular uptake experiments demonstrated that the introduction of HA to the NP surface endowed NPs with colon cancer-targeting capability and markedly increased cellular uptake efficiency compared with chitosan-coated NPs. Importantly, the combined delivery of CPT and CUR in one HA-functionalized NP exerted strong synergistic effects. HA-CPT/CUR-NP (1 : 1) showed the highest antitumor activity among the three HA-CPT/CUR-NPs, resulting in an extremely low combination index. Collectively, our findings indicate that this HA-CPT/CUR-NP can be exploited as an efficient formulation for colon cancer-targeted combination chemotherapy.
Figures





Similar articles
-
Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy.J Mater Chem B. 2015 Oct 21;3(39):7724-7733. doi: 10.1039/c5tb01245g. Epub 2015 Sep 1. J Mater Chem B. 2015. PMID: 26617985 Free PMC article.
-
Chondroitin sulfate-functionalized polymeric nanoparticles for colon cancer-targeted chemotherapy.Colloids Surf B Biointerfaces. 2019 May 1;177:399-406. doi: 10.1016/j.colsurfb.2019.02.031. Epub 2019 Feb 16. Colloids Surf B Biointerfaces. 2019. PMID: 30785037
-
Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells.Int J Nanomedicine. 2014 Feb 21;9:1083-96. doi: 10.2147/IJN.S59779. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24591829 Free PMC article.
-
Development and statistical optimization of camptothecin loaded hyaluronic acid and zein polymeric nanoparticles towards the treatment of melanoma.Int J Biol Macromol. 2025 Sep;321(Pt 2):146330. doi: 10.1016/j.ijbiomac.2025.146330. Epub 2025 Jul 25. Int J Biol Macromol. 2025. PMID: 40716545
-
Advanced drug delivery systems of curcumin for cancer chemoprevention.Cancer Prev Res (Phila). 2011 Aug;4(8):1158-71. doi: 10.1158/1940-6207.CAPR-10-0006. Epub 2011 May 5. Cancer Prev Res (Phila). 2011. PMID: 21546540 Free PMC article. Review.
Cited by
-
Overcoming Multidrug Resistance in Bacteria Through Antibiotics Delivery in Surface-Engineered Nano-Cargos: Recent Developments for Future Nano-Antibiotics.Front Bioeng Biotechnol. 2021 Jul 8;9:696514. doi: 10.3389/fbioe.2021.696514. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34307323 Free PMC article. Review.
-
Ligand-based targeted therapy: a novel strategy for hepatocellular carcinoma.Int J Nanomedicine. 2016 Oct 31;11:5645-5669. doi: 10.2147/IJN.S115727. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27920520 Free PMC article. Review.
-
Nanomaterials targeting cancer stem cells to overcome drug resistance and tumor recurrence.Front Oncol. 2025 Jun 6;15:1499283. doi: 10.3389/fonc.2025.1499283. eCollection 2025. Front Oncol. 2025. PMID: 40548119 Free PMC article. Review.
-
Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy.Expert Opin Drug Deliv. 2017 Jan;14(1):65-73. doi: 10.1080/17425247.2016.1205583. Epub 2016 Jul 6. Expert Opin Drug Deliv. 2017. PMID: 27337289 Free PMC article. Review.
-
Low-frequency ultrasound may improve drug penetration in colonic mucosa.Transl Cancer Res. 2017 Mar;6(Suppl 2):S276-S279. doi: 10.21037/tcr.2017.03.62. Transl Cancer Res. 2017. PMID: 30581770 Free PMC article. No abstract available.
References
-
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kröning H, Maroun J, Marschner N, McKendrick J, Pawlicki M, Rosso R, Schüller J, Seitz J, Stabuc B, Tujakowski J, Hazel GV, Zaluski J, Scheithauer W. N. Engl. J. Med. 2005;352:2696. - PubMed
-
- Terzic J, Grivennikov S, Karin E, Karin M. Gastroenterology. 2010;138:2101. - PubMed
-
- Liu C, Zhao G, Liu J, Ma N, Chivukula P, Perelman L, Okada K, Chen Z, Gough D, Yu L. J. Control. Release. 2009;140:277. - PubMed
-
- Lane D. Nat. Biotechnol. 2006;24:163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

