Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II
- PMID: 26455390
- PMCID: PMC4609301
- DOI: 10.1016/j.molcel.2015.09.006
Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II
Abstract
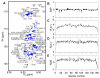
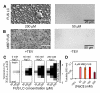
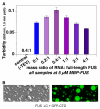
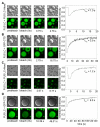
Phase-separated states of proteins underlie ribonucleoprotein (RNP) granules and nuclear RNA-binding protein assemblies that may nucleate protein inclusions associated with neurodegenerative diseases. We report that the N-terminal low-complexity domain of the RNA-binding protein Fused in Sarcoma (FUS LC) is structurally disordered and forms a liquid-like phase-separated state resembling RNP granules. This state directly binds the C-terminal domain of RNA polymerase II. Phase-separated FUS lacks static structures as probed by fluorescence microscopy, indicating they are distinct from both protein inclusions and hydrogels. We use solution nuclear magnetic resonance spectroscopy to directly probe the dynamic architecture within FUS liquid phase-separated assemblies. Importantly, we find that FUS LC retains disordered secondary structure even in the liquid phase-separated state. Therefore, we propose that disordered protein granules, even those made of aggregation-prone prion-like domains, are dynamic and disordered molecular assemblies with transiently formed protein-protein contacts.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- P30GM103410/GM/NIGMS NIH HHS/United States
- P20 GM103430/GM/NIGMS NIH HHS/United States
- P30 GM103410/GM/NIGMS NIH HHS/United States
- P20GM103430/GM/NIGMS NIH HHS/United States
- P20 GM104937/GM/NIGMS NIH HHS/United States
- S10RR020923/RR/NCRR NIH HHS/United States
- P30RR031153/RR/NCRR NIH HHS/United States
- P30 RR031153/RR/NCRR NIH HHS/United States
- P20GM104937/GM/NIGMS NIH HHS/United States
- S10 RR020923/RR/NCRR NIH HHS/United States
- S10RR02763/RR/NCRR NIH HHS/United States
- P20 RR018728/RR/NCRR NIH HHS/United States
- P20RR018728/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

