Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease
- PMID: 26455458
- PMCID: PMC4826627
- DOI: 10.1016/j.pneurobio.2015.09.012
Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease
Abstract
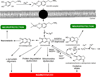
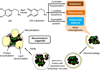
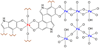
There are several interrelated mechanisms involving iron, dopamine, and neuromelanin in neurons. Neuromelanin accumulates during aging and is the catecholamine-derived pigment of the dopamine neurons of the substantia nigra and norepinephrine neurons of the locus coeruleus, the two neuronal populations most targeted in Parkinson's disease. Many cellular redox reactions rely on iron, however an altered distribution of reactive iron is cytotoxic. In fact, increased levels of iron in the brain of Parkinson's disease patients are present. Dopamine accumulation can induce neuronal death; however, excess dopamine can be removed by converting it into a stable compound like neuromelanin, and this process rescues the cell. Interestingly, the main iron compound in dopamine and norepinephrine neurons is the neuromelanin-iron complex, since neuromelanin is an effective metal chelator. Neuromelanin serves to trap iron and provide neuronal protection from oxidative stress. This equilibrium between iron, dopamine, and neuromelanin is crucial for cell homeostasis and in some cellular circumstances can be disrupted. Indeed, when neuromelanin-containing organelles accumulate high load of toxins and iron during aging a neurodegenerative process can be triggered. In addition, neuromelanin released by degenerating neurons activates microglia and the latter cause neurons death with further release of neuromelanin, then starting a self-propelling mechanism of neuroinflammation and neurodegeneration. Considering the above issues, age-related accumulation of neuromelanin in dopamine neurons shows an interesting link between aging and neurodegeneration.
Keywords: Dopamine; Human neuromelanin; Iron; Melanin; Parkinson's disease.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Abbas N, Lücking CB, Ricard S, Dürr A, Bonifati V, De Michele G, Bouley S, Vaughan JR, Gasser T, Marconi R, Broussolle E, Brefel-Courbon C, Harhangi BS, Oostra BA, Fabrizio E, Böhme GA, Pradier L, Wood NW, Filla A, Meco G, Denefle P, Agid Y, Brice A. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum. Mol. Genet. 1999;8:567–574. - PubMed
-
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. - PubMed
-
- Abdel-Malek Z, Kadekaro AL. Human Pigmentation: Its regulation by Ultraviolet Light and by Endocrine, Paracrine and Autocrine Factors. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 410–420.
-
- Agrup G, Hansson C, Rorsman H, Rosengren E. The effect of cysteine on oxidation of tyrosine, dopa, and cysteinyldopas. Arch. Dermatol. Res. 1982;272:103–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

