PET imaging evaluation of [(18)F]DBT-10, a novel radioligand specific to α7 nicotinic acetylcholine receptors, in nonhuman primates
- PMID: 26455500
- PMCID: PMC4733418
- DOI: 10.1007/s00259-015-3209-0
PET imaging evaluation of [(18)F]DBT-10, a novel radioligand specific to α7 nicotinic acetylcholine receptors, in nonhuman primates
Abstract
Purpose: Positron emission tomography (PET) radioligands specific to α7 nicotinic acetylcholine receptors (nAChRs) afford in vivo imaging of this receptor for neuropathologies such as Alzheimer's disease, schizophrenia, and substance abuse. This work aims to characterize the kinetic properties of an α7-nAChR-specific radioligand, 7-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-2-[(18)F]-fluorodibenzo[b,d]thiophene 5,5-dioxide ([(18)F]DBT-10), in nonhuman primates.
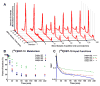
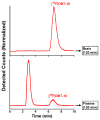
Methods: [(18)F]DBT-10 was produced via nucleophilic substitution of the nitro-precursor. Four Macaca mulatta subjects were imaged with [(18)F]DBT-10 PET, with measurement of [(18)F]DBT-10 parent concentrations and metabolism in arterial plasma. Baseline PET scans were acquired for all subjects. Following one scan, ex vivo analysis of brain tissue was performed to inspect for radiolabeled metabolites in brain. Three blocking scans with 0.69 and 1.24 mg/kg of the α7-nAChR-specific ligand ASEM were also acquired to assess dose-dependent blockade of [(18)F]DBT-10 binding. Kinetic analysis of PET data was performed using the metabolite-corrected input function to calculate the parent fraction corrected total distribution volume (V T/f P).
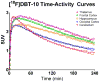
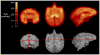
Results: [(18)F]DBT-10 was produced within 90 min at high specific activities of 428 ± 436 GBq/μmol at end of synthesis. Metabolism of [(18)F]DBT-10 varied across subjects, stabilizing by 120 min post-injection at parent fractions of 15-55%. Uptake of [(18)F]DBT-10 in brain occurred rapidly, reaching peak standardized uptake values (SUVs) of 2.9-3.7 within 30 min. The plasma-free fraction was 18.8 ± 3.4%. No evidence for radiolabeled [(18)F]DBT-10 metabolites was found in ex vivo brain tissue samples. Kinetic analysis of PET data was best described by the two-tissue compartment model. Estimated V T/f P values were 193-376 ml/cm(3) across regions, with regional rank order of thalamus > frontal cortex > striatum > hippocampus > occipital cortex > cerebellum > pons. Dose-dependent blockade of [(18)F]DBT-10 binding by structural analog ASEM was observed throughout the brain, and occupancy plots yielded a V ND/f P estimate of 20 ± 16 ml/cm(3).
Conclusion: These results demonstrate suitable kinetic properties of [(18)F]DBT-10 for in vivo quantification of α7-nAChR binding in nonhuman primates.
Keywords: Alpha 7; Nicotine; Nicotinic acetylcholine receptor; PET.
Figures



Similar articles
-
PET imaging of α7 nicotinic acetylcholine receptors: a comparative study of [18F]ASEM and [18F]DBT-10 in nonhuman primates, and further evaluation of [18F]ASEM in humans.Eur J Nucl Med Mol Imaging. 2017 Jun;44(6):1042-1050. doi: 10.1007/s00259-017-3621-8. Epub 2017 Jan 24. Eur J Nucl Med Mol Imaging. 2017. PMID: 28120003 Free PMC article.
-
Derivatives of dibenzothiophene for positron emission tomography imaging of α7-nicotinic acetylcholine receptors.J Med Chem. 2013 Oct 10;56(19):7574-89. doi: 10.1021/jm401184f. Epub 2013 Sep 19. J Med Chem. 2013. PMID: 24050653 Free PMC article.
-
18F-ASEM, a radiolabeled antagonist for imaging the α7-nicotinic acetylcholine receptor with PET.J Nucl Med. 2014 Apr;55(4):672-7. doi: 10.2967/jnumed.113.132068. Epub 2014 Feb 20. J Nucl Med. 2014. PMID: 24556591 Free PMC article.
-
Development of [(18)F]ASEM, a specific radiotracer for quantification of the α7-nAChR with positron-emission tomography.Biochem Pharmacol. 2015 Oct 15;97(4):566-575. doi: 10.1016/j.bcp.2015.07.030. Epub 2015 Jul 29. Biochem Pharmacol. 2015. PMID: 26232729 Free PMC article. Review.
-
Advances in PET Imaging of α7 Nicotinic Receptors: From Radioligand Development to CNS Applications.Basic Clin Pharmacol Toxicol. 2025 Apr;136(4):e70025. doi: 10.1111/bcpt.70025. Basic Clin Pharmacol Toxicol. 2025. PMID: 40084546 Free PMC article. Review.
Cited by
-
Positron Emission Tomography in Animal Models of Tauopathies.Front Aging Neurosci. 2022 Jan 10;13:761913. doi: 10.3389/fnagi.2021.761913. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35082657 Free PMC article. Review.
-
Radioligand binding analysis of α 2 adrenoceptors with [11C]yohimbine in brain in vivo: Extended Inhibition Plot correction for plasma protein binding.Sci Rep. 2017 Nov 22;7(1):15979. doi: 10.1038/s41598-017-16020-1. Sci Rep. 2017. PMID: 29167492 Free PMC article.
-
In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors.Molecules. 2020 Mar 20;25(6):1425. doi: 10.3390/molecules25061425. Molecules. 2020. PMID: 32245032 Free PMC article.
-
Relative Strengths of Three Linearizations of Receptor Availability: Saturation, Inhibition, and Occupancy Plots.J Nucl Med. 2022 Feb;63(2):294-301. doi: 10.2967/jnumed.117.204453. Epub 2021 Jun 4. J Nucl Med. 2022. PMID: 34088774 Free PMC article.
-
Current radiotracers to image neurodegenerative diseases.EJNMMI Radiopharm Chem. 2019 Jul 26;4(1):17. doi: 10.1186/s41181-019-0070-7. EJNMMI Radiopharm Chem. 2019. PMID: 31659510 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

