The human Kell blood group binds the erythroid 4.1R protein: new insights into the 4.1R-dependent red cell membrane complex
- PMID: 26455906
- PMCID: PMC4715498
- DOI: 10.1111/bjh.13778
The human Kell blood group binds the erythroid 4.1R protein: new insights into the 4.1R-dependent red cell membrane complex
Abstract
Protein 4.1R plays an important role in maintaining the mechanical properties of the erythrocyte membrane. We analysed the expression of Kell blood group protein in erythrocytes from a patient with hereditary elliptocytosis associated with complete 4.1R deficiency (4.1(-) HE). Flow cytometry and Western blot analyses revealed a severe reduction of Kell. In vitro pull down and co-immunoprecipitation experiments from erythrocyte membranes showed a direct interaction between Kell and 4.1R. Using different recombinant domains of 4.1R and the cytoplasmic domain of Kell, we demonstrated that the R(46) R motif in the juxta-membrane region of Kell binds to lobe B of the 4.1R FERM domain. We also observed that 4.1R deficiency is associated with a reduction of XK and DARC (also termed ACKR1) proteins, the absence of the glycosylated form of the urea transporter B and a slight decrease of band 3. The functional alteration of the 4.1(-) HE erythrocyte membranes was also determined by measuring various transport activities. We documented a slower rate of HCO3 (-) /Cl(-) exchange, but normal water and ammonia transport across erythrocyte membrane in the absence of 4.1. These findings provide novel insights into the structural organization of blood group antigen proteins into the 4.1R complex of the human red cell membrane.
Keywords: 4.1R; Kell protein; blood group antigens; erythrocyte membrane; macromolecular complex.
© 2015 John Wiley & Sons Ltd.
Figures


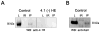
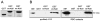


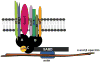
References
-
- An XL, Takakuwa Y, Manno S, Han BG, Gascard P, Mohandas N. Structural and functional characterization of protein 4.1R-phosphatidylserine interaction: potential role in 4.1R sorting within cells. J Biol Chem. 2001;276:35778–35785. - PubMed
-
- Anderson RA, Lovrien RE. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984;307:655–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

