Downregulation of the Canonical WNT Signaling Pathway by TGFβ1 Inhibits Photoreceptor Differentiation of Adult Human Müller Glia with Stem Cell Characteristics
- PMID: 26456050
- PMCID: PMC4692127
- DOI: 10.1089/scd.2015.0262
Downregulation of the Canonical WNT Signaling Pathway by TGFβ1 Inhibits Photoreceptor Differentiation of Adult Human Müller Glia with Stem Cell Characteristics
Abstract
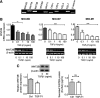
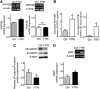
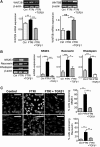
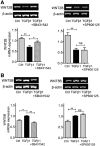
Müller glia are responsible for the retina regeneration observed in zebrafish. Although the human retina harbors Müller glia with stem cell characteristics, there is no evidence that they regenerate the retina after disease or injury. Transforming growth factor-β (TGFβ) and Wnt signaling regulate retinal neurogenesis and inflammation, but their roles in the neural differentiation of human Müller stem cells (hMSC) are not known. We examined hMSC lines in vitro for the expression of various Wnt signaling components and for their modulation by TGFβ1, as well as the effect of this cytokine on the photoreceptor differentiation of these cells. Culture of hMSC with a combination of factors that induce photoreceptor differentiation of hMSC (FGF2, taurine, retinoic acid, and insulin-like growth factor type1; FTRI), markedly upregulated the expression of components of the canonical Wnt signaling pathway, including WNT2B, DKK1, and active β-CATENIN. Although FTRI did not modify mRNA expression of WNT5B, a component of the noncanonical/planar cell polarity Wnt pathway, it upregulated its secretion. Furthermore, TGFβ1 not only decreased WNT2B expression, but also inhibited FTRI-induced photoreceptor differentiation of hMSC, as determined by expression of the photoreceptor markers NR2E3, RHODOPSIN, and RECOVERIN. Inhibition of TGFβ1 signaling by an ALK5 inhibitor prevented TGFβ1-induced changes in the expression of the two Wnt ligands examined. More importantly, inhibition of the canonical WNT signaling by XAV-939 prevented FTRI-induced photoreceptor differentiation. These observations suggest that TGFβ may play a key role in preventing neural differentiation of hMSC and may constitute a potential target for induction of endogenous regeneration of the human retina.
Figures




References
-
- Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, Khaw PT. and Limb GA. (2007). MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells 25:2033–2043 - PubMed
-
- Singhal S, Bhatia B, Jayaram H, Becker S, Jones MF, Cottrill PB, Khaw PT, Salt TE. and Limb GA. (2012). Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med 1:188–199 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
