Synergistic anti-myeloma activity of the proteasome inhibitor marizomib and the IMiD immunomodulatory drug pomalidomide
- PMID: 26456076
- PMCID: PMC4715649
- DOI: 10.1111/bjh.13780
Synergistic anti-myeloma activity of the proteasome inhibitor marizomib and the IMiD immunomodulatory drug pomalidomide
Abstract
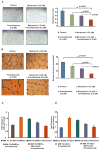
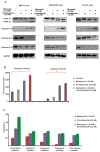
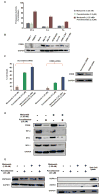
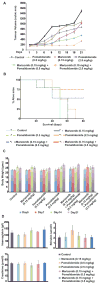
The proteasome inhibitor bortezomib is an effective therapy for the treatment of relapsed and refractory multiple myeloma (RRMM); however, prolonged treatment can be associated with toxicity, peripheral neuropathy and drug resistance. Our earlier studies showed that the novel proteasome inhibitor marizomib is distinct from bortezomib in its chemical structure, mechanisms of action and effects on proteasomal activities, and that it can overcome bortezomib resistance. Pomalidomide, like lenalidomide, has potent immunomodulatory activity and has been approved by the US Food and Drug Administration for the treatment of RRMM. Here, we demonstrate that combining low concentrations of marizomib with pomalidomide induces synergistic anti-MM activity. Marizomib plus pomalidomide-induced apoptosis is associated with: (i) activation of caspase-8, caspase-9, caspase-3 and PARP cleavage, (ii) downregulation of cereblon (CRBN), IRF4, MYC and MCL1, and (iii) suppression of chymotrypsin-like, caspase-like, and trypsin-like proteasome activities. CRBN-siRNA attenuates marizomib plus pomalidomide-induced MM cells death. Furthermore, marizomib plus pomalidomide inhibits the migration of MM cells and tumour-associated angiogenesis, as well as overcomes cytoprotective effects of bone marrow microenvironment. In human MM xenograft model studies, the combination of marizomib and pomalidomide is well tolerated, inhibits tumour growth and prolongs survival. These preclinical studies provide the rationale for on-going clinical trials of combined marizomib and pomalidomide to improve outcome in patients with RRMM.
Keywords: cancer and drug therapy; marizomib; multiple myeloma; pomalidomide.
© 2015 John Wiley & Sons Ltd.
Conflict of interest statement
Figures

References
-
- Anderson KC. Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol. 2007;35:155–162. - PubMed
-
- Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, Leyvraz S, Michallet M, Yakoub-Agha I, Garderet L, Marit G, Michaux L, Voillat L, Renaud M, Grosbois B, Guillerm G, Benboubker L, Monconduit M, Thieblemont C, Casassus P, Caillot D, Stoppa AM, Sotto JJ, Wetterwald M, Dumontet C, Fuzibet JG, Azais I, Dorvaux V, Zandecki M, Bataille R, Minvielle S, Harousseau JL, Facon T, Mathiot C. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

