Replication stress caused by low MCM expression limits fetal erythropoiesis and hematopoietic stem cell functionality
- PMID: 26456157
- PMCID: PMC4608254
- DOI: 10.1038/ncomms9548
Replication stress caused by low MCM expression limits fetal erythropoiesis and hematopoietic stem cell functionality
Abstract
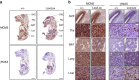
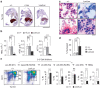
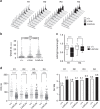
Replicative stress during embryonic development influences ageing and predisposition to disease in adults. A protective mechanism against replicative stress is provided by the licensing of thousands of origins in G1 that are not necessarily activated in the subsequent S-phase. These 'dormant' origins provide a backup in the presence of stalled forks and may confer flexibility to the replication program in specific cell types during differentiation, a role that has remained unexplored. Here we show, using a mouse strain with hypomorphic expression of the origin licensing factor mini-chromosome maintenance (MCM)3 that limiting origin licensing in vivo affects the functionality of hematopoietic stem cells and the differentiation of rapidly-dividing erythrocyte precursors. Mcm3-deficient erythroblasts display aberrant DNA replication patterns and fail to complete maturation, causing lethal anemia. Our results indicate that hematopoietic progenitors are particularly sensitive to replication stress, and full origin licensing ensures their correct differentiation and functionality.
Figures



References
-
- Masai H., Matsumoto S., You Z., Yoshizawa-Sugata N. & Oda M. Eukaryotic chromosome DNA replication: where, when and how? Annu. Rev. Biochem. 79, 89–130 (2010). - PubMed
-
- Bicknell L. S. et al.. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat. Genet. 43, 350–355 (2011a). - PubMed
-
- Guernsey D. L. et al.. Mutations in origin recognition complex gene ORC4 cause Meier–Gorlin syndrome. Nat. Genet. 43, 360–364 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

