A Recurrent Mutation in CACNA1G Alters Cav3.1 T-Type Calcium-Channel Conduction and Causes Autosomal-Dominant Cerebellar Ataxia
- PMID: 26456284
- PMCID: PMC4667105
- DOI: 10.1016/j.ajhg.2015.09.007
A Recurrent Mutation in CACNA1G Alters Cav3.1 T-Type Calcium-Channel Conduction and Causes Autosomal-Dominant Cerebellar Ataxia
Abstract
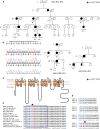
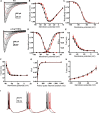
Hereditary cerebellar ataxias (CAs) are neurodegenerative disorders clinically characterized by a cerebellar syndrome, often accompanied by other neurological or non-neurological signs. All transmission modes have been described. In autosomal-dominant CA (ADCA), mutations in more than 30 genes are implicated, but the molecular diagnosis remains unknown in about 40% of cases. Implication of ion channels has long been an ongoing topic in the genetics of CA, and mutations in several channel genes have been recently connected to ADCA. In a large family affected by ADCA and mild pyramidal signs, we searched for the causative variant by combining linkage analysis and whole-exome sequencing. In CACNA1G, we identified a c.5144G>A mutation, causing an arginine-to-histidine (p.Arg1715His) change in the voltage sensor S4 segment of the T-type channel protein Cav3.1. Two out of 479 index subjects screened subsequently harbored the same mutation. We performed electrophysiological experiments in HEK293T cells to compare the properties of the p.Arg1715His and wild-type Cav3.1 channels. The current-voltage and the steady-state activation curves of the p.Arg1715His channel were shifted positively, whereas the inactivation curve had a higher slope factor. Computer modeling in deep cerebellar nuclei (DCN) neurons suggested that the mutation results in decreased neuronal excitability. Taken together, these data establish CACNA1G, which is highly expressed in the cerebellum, as a gene whose mutations can cause ADCA. This is consistent with the neuropathological examination, which showed severe Purkinje cell loss. Our study further extends our knowledge of the link between calcium channelopathies and CAs.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. - PubMed
-
- Hersheson J., Haworth A., Houlden H. The inherited ataxias: genetic heterogeneity, mutation databases, and future directions in research and clinical diagnostics. Hum. Mutat. 2012;33:1324–1332. - PubMed
-
- Coutelier M., Stevanin G., Brice A. Genetic landscape remodelling in spinocerebellar ataxias: the influence of next-generation sequencing. J. Neurol. 2015 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

